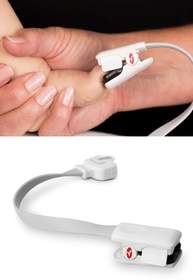
NEUCHATEL, SWITZERLAND--(Marketwired - August 28, 2014) - Masimo (NASDAQ: MASI) today announced CE Mark, clearance in Japan, and limited market release of the rainbow® DCI-mini™, the first noninvasive haemoglobin (SpHb®) spot-check sensor for infants and small children (weight 3 to 30 kg). Paired with Masimo's handheld Pronto® device, the rainbow® DCI-mini sensors are designed to help clinicians quickly and easily spot-check haemoglobin levels, which may facilitate the identification of anaemia -- a condition reflected by low haemoglobin when there are not enough red blood cells carrying oxygen to the tissues. In low-resource countries, an estimated 3.5 billion people are anaemic,(1) making it one of the world's most common disorders.
Previously, SpHb spot-check sensors were available only for patients weighing 10 kg or more. Because iron deficient-anaemia during infancy and childhood has been shown to have long-lasting adverse effects on neurodevelopment,(2) international health organizations recommend screening infants for anaemia between the ages of 9 to 12 months -- an age that is often below 10 kg -- with additional screening between the ages of 1 and 5 years for patients at risk.(3,4)
Masimo's rainbow® DCI-mini is a versatile sensor that is designed to facilitate proper placement and ease of use for clinicians and patients. The sensor uses a lightweight ribbon cable to connect to the Pronto device and a digit clip that is applied to a small child's finger or on an infant's big toe or thumb. The new rainbow® DCI-mini reusable spot-check sensor is ideal for clinics, public health programmes, and hospital emergency departments.
"The conventional process of drawing blood is a traumatic event, especially for younger patients, not to mention the cost," said Dr. Mohammed Bailony, an M.D. in paediatrics, who practices paediatric haematology-oncology at several hospitals in San Diego, including Scripps Mercy Hospital and Rady Children's Hospital. Dr. Bailony has screened patients for anaemia in developing nations and has used the rainbow® DCI-mini in clinical trials.
"SpHb assessment with the DCI-mini definitely will have a big role in developing nations, where laboratories are often not available," Dr. Bailony said. "In small cities, rural areas, and for mobile clinics, this will be a tremendous help in assessing patients. It's fast. It's friendly. And unlike needles, I haven't seen a kid who's scared of it."
"The DCI-mini allows clinicians and public health programmes around the world to expand haemoglobin assessment to vulnerable populations that need a noninvasive and convenient method," said Masimo founder and CEO Joe Kiani. "Every 90 seconds a women dies from complications due to pregnancy and many of them due to anaemia. In addition children under 5 years old who suffer from anaemia do not develop fully, which has long term implications to the lives of those afflicted, as well as society in general. We hope that the new rainbow DCI-mini will help more infants and small children, along with their moms, around the globe to receive timely assessment and treatment, which will benefit their long-term health, as well as the health of our society."
The DCI-mini spot-check SpHb sensor is available in Europe, Japan and many other countries, but not available for sale in the United States, Canada, China, Singapore, Brazil and Mexico. For a more detailed list of country exceptions and available markets for sale, contact Masimo Customer Service.
1 United Nations Administrative Committee on Coordination Nutrition (ACC/SCN) 4TH Report on the world nutrition situation: Nutrition Throughout the Life Cycle. Sub-Committee on Geneva: ACC/SCN; 2000.
2 Baker R, Greer R, The Committee on Nutrition, "Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0-3 Years of Age)" PEDIATRICS Vol. 126 No. 5 November 1, 2010 pp. 1040 -1050
3 United Nations Children's fund, United Nations University and World Health Organization, "Iron Deficiency Anaemia, Assessment, Prevention, and Control - A Guide for Programme Managers" 2001: WHO/NHD/01.3. Available here
4 Kohli-Kumar M, "Screening for Anemia in Children: AAP Recommendations--A Critique" PEDIATRICS Vol. 108 No. 3 September 1, 2001 pp. e56
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care--helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb accurately tracks and trends Hb changes in all patients, risks related to our belief that SpHb enables quick and easy noninvasive spot-checking of hemoglobin at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
Help employers find you! Check out all the jobs and post your resume.