TUCSON, Ariz., May 8, 2014 /PRNewswire/ -- Randy Shepherd's fascinating story of life with a SynCardia temporary Total Artificial Heart has touched the hearts of many who have found inspiration as well as information on how the world's first and only FDA, Health Canada and CE approved Total Artificial Heart saves lives. The SynCardia Heart is used as a bridge to transplant to a donor heart for patients with end-stage biventricular (both sides) heart failure.
To view the multimedia assets associated with this release, please click: http://www.multivu.com/mnr/7043351-syncardia-total-artificial-heart-detailed-on-social-media-site

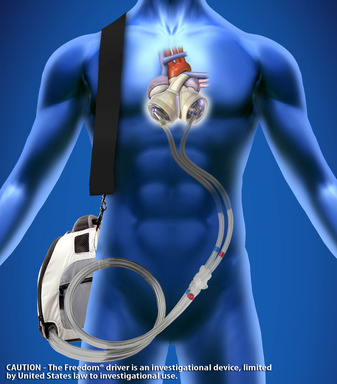
Shepherd, 40, received a SynCardia Total Artificial Heart on June 20, 2013. His heart had been damaged by two bouts of rheumatic fever when he was a child. After receiving the SynCardia Heart, Randy recovered. When his clinical condition stabilized, he was equipped with a Freedom® portable driver that allows SynCardia Heart patients nearly unlimited mobility.
Like a heart transplant, the SynCardia Heart replaces both failing heart ventricles and the four heart valves. It is the only approved device that eliminates the symptoms and source of end-stage biventricular heart failure in which the heart's ventricles can no longer pump enough blood for the person to survive.
Data published in the New England Journal of Medicine from the 10-year pivotal clinical study that led to FDA approval showed that 79% of patients who received the SynCardia Total Artificial Heart were bridged to transplant. This is the highest bridge to transplant rate for any approved heart device in the world.
Shepherd, a Mesa, Arizona resident, walked 4.2 miles in the April 26, 2014, Pat's Run that honors fallen soldier Pat Tillman. Proceeds from the race directly support scholarships for Tillman Military Scholars. Randy became the first person without a human heart to complete the long-distance Tempe, Arizona course. To power his SynCardia Total Artificial Heart, Randy carried the 13.5-pound Freedom portable driver in a backpack.
"I am hoping by next year at Pat's Run I'll be running it instead of walking it," he says. "And I am hoping to have a donor heart. But if not, I'll be back there with my backpack again and I'm going to do it again next year."
Three days later Shepherd started an “Ask Me Anything” (AMA) interview on Reddit, a social media website. He responded with humor, patience and openness to hundreds of questions and comments. They covered the science of the SynCardia Heart, the philosophy of living without a human heart and the day-to-day practicalities of living with the SynCardia Total Artificial Heart powered by the Freedom portable driver. It provides an insider's look into what life is like for a SynCardia Heart patient.
While the SynCardia Heart currently is not approved to permanently replace the heart, SynCardia is working with regulatory authorities to study and gain approval for what is called destination therapy so patients who do not qualify for, or want, a donor human heart can live with the SynCardia Total Artificial Heart.
Shepherd, whose Pat's Run feat also has been the subject of local and national media stories, has inspired some of the interviewers on his AMA to sign up as heart and organ donors. He supports, encourages and consoles those facing medical challenges.
In a response to one comment, Shepherd says: "I have had so many people from whom I have received inspiration. I am glad to think that I could be that for someone else."
For additional information, please visit: http://www.syncardia.com/
Like SynCardia on Facebook
Follow SynCardia on Twitter @SynCardia
Connect with SynCardia on LinkedIn
Share and Discover on Google+
CAUTION The Freedom portable driver is an investigational device, limited by United States law to investigational use.
About the SynCardia temporary Total Artificial Heart
SynCardia Systems, Inc. (Tucson, AZ) is the privately-held manufacturer of the world's first and only FDA, Health Canada and CE approved Total Artificial Heart. Originally used as a permanent replacement heart, the SynCardia Total Artificial Heart is currently approved as a bridge to transplant for people suffering from end stage heart failure affecting both sides of the heart (biventricular failure).
More than 400 SynCardia Total Artificial Heart implants have been performed since January 2011. The SynCardia Heart has provided almost 145,000 patient days of support from over 1,300 implants. The youngest patient to receive a SynCardia Heart was 9 years old; the oldest was 76 years old.
Similar to a heart transplant, the SynCardia Total Artificial Heart replaces both failing heart ventricles and the four heart valves. It is the only device that eliminates the symptoms and source of end stage biventricular failure. Unlike a donor heart, the Total Artificial Heart is immediately available at 97 SynCardia Certified Centers worldwide with 39 others in the process of certification.
The Total Artificial Heart provides immediate, safe blood flow of up to 9.5 liters per minute through each ventricle. This high volume of blood flow helps speed the recovery of vital organs, helping make the patient a better transplant candidate.



To view the multimedia assets associated with this release, please click: http://www.multivu.com/mnr/7043351-syncardia-total-artificial-heart-detailed-on-social-media-site
SOURCE SynCardia Systems, Inc.
Help employers find you! Check out all the jobs and post your resume.




