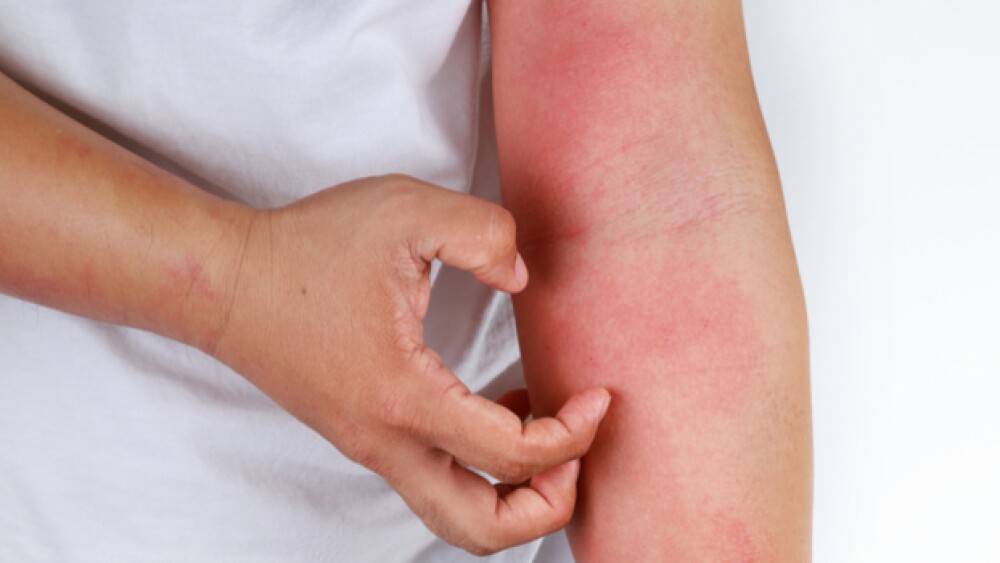
While waiting for further updates, here’s everything you need to know about this highly-anticipated atopic dermatitis treatment.
Will Leo Pharma’s Adtralza® (tralokinumab) get its U.S. Food and Drug Administration (FDA) approval this year? While there’s no confirmation yet, the pharmaceutical company has already received a Positive CHMP Opinion for the treatment.
While waiting for further updates, here’s everything you need to know about this highly-anticipated atopic dermatitis treatment.
RELATED: Atopic Dermatitis Space Will Heat Up in 2020, Analyst Predicts
What is Tralokinumab?
Tralokinumab is not the first treatment for atopic dermatitis. In fact, the FDA recently approved Dupilumab for public consumption. However, Tralokinumab is said to be a better treatment given the side effects of Dupilumab.
Specifically, Tralokinumab is a human monoclonal antibody that is said to be the treatment for asthma and other inflammatory diseases related to eczema or atopic dermatitis (AD). The antibody targets the cytokine interleukin 13 (IL-13), which is the main reason for inflammation in AD. It prevents IL-13 from binding to IL-13Ra1 and IL-13Ra2.
Other Atopic Dermatitis Treatments
Lebrikizumab, on the other hand, is a different human monoclonal antibody. The soluble IL-13 content prevents IL‐4Rα/IL‐13Rα1 heterodimerization and blocks downstream signaling.
In a clinical test, both Tralokinumab and Lebrikizumab yielded positive results, becoming the most acceptable and most effective treatments for patients with moderate to severe eczema.
Tralokinumab and Lebrikizumab are not the pioneer treatments for AD. The first approved treatment was Dupilumab. Unfortunately, it led to conjunctivitis in some patients (8-38%), while others developed neck and head dermatitis. This paved the way for the development of other treatment options.
What Are the Side Effects of Tralokinumab, Lebrikizumab, and Dupilumab?
While the first treatment in Dupilumab had adverse side effects on some people, Tralokinumab and Lebrikizumab proved to have minimal side effects.
Side Effects of Tralokinumab
In a study to see the efficacy of this treatment, 17.6% of the participants experienced headache and upper respiratory tract infection as side effects.
Reactions at the injection site were felt by 5.2% of the group injected with Tralokinumab. In comparison, 3.9% of the people from the placebo group experienced reactions.
At the same time, conjunctivitis was also experienced by 2% of the Tralokinumab group who had a 45 mg dose, 5.9% who had the 150 mg dose, and 3.9% in the placebo group.
Side Effects of Lebrikizumab
In a different study, Lebrikizumab pointed out similar side effects seen in the Tralokinumab trial.
Injection site pain was felt by 5.3%, injection site reaction experienced by 1.3%, headache was felt by 1.3-5.3%, nasopharyngitis felt by 2.5-12%, and upper respiratory tract infection experienced by 2.7-11.3%.
Side Effects of Dupilumab
While Dupilumab is now available in the market as Dupixent, common side effects include conjunctivitis, oral herpes, reactions in the site injection, swelling of the eyes, inflammation of the cornea, drying of the eyes and eye itching.
Is Tralokinumab FDA Approved?
In July of 2020, the FDA accepted the application for a biologics license from LEO Pharma for tralokinumab. The FDA recently reviewed the efficacy of the treatment after it underwent a Biological License application, but the result of its approval is still pending.
Meanwhile, the pharmaceutical company presented a long-term safety check and efficacy data, which the agency recognizes.
“The FDA has not raised any questions to the clinical efficacy or safety of tralokinumab, but only requested additional data relating to a device component of the combination product,” EVP for Global R&D at LEO Pharma Jörg Möller claimed. ”We will now work closely with the FDA to address their request and bring tralokinumab to the U.S. patients as quickly as possible.”
Meanwhile, the U.K. regulator has its thumbs up for approval of the treatment and is a step closer to being accepted.
To answer the question if the FDA approves Tralokinumab, the current answer is no, but given the positive feedback from the recent data, chances are that it’s only a matter of time for Leo Pharma’s Tralokinumab to be approved.
On the other hand, Lebrikizumab is on its Phase IIb of its clinical trial and is expected to soon roll out its third phase.
“Lebrikizumab is a potent and specific inhibitor of IL-13 with a differentiated mechanism of action and attractive pharmacokinetic properties,” chief medical officer of Dermira Eugene Bauer claimed. “Data from preclinical and clinical studies, including pharmacokinetic and pharmacodynamic results from early clinical experience in atopic dermatitis, are encouraging and suggest higher doses of lebrikizumab could lead to greater efficacy in atopic dermatitis, while potentially offering a less frequent and therefore more convenient dosing regimen relative to existing therapies. If successfully developed, we believe that lebrikizumab could be a best-in-class IL-13 inhibitor and could have a best-in-disease profile.”
What is Atopic Dermatitis?
To better understand Tralokinumab and other treatments, it is best to know the skin condition it aims to cure.
Often referred to as eczema, atopic dermatitis is a skin condition that causes redness and itching in people.
The types of AD often experienced by people with this condition include hand eczema, contact dermatitis, and dyshidrotic eczema. These conditions are classified as moderate to severe forms of AD.
Contact dermatitis is triggered when the skin of a person comes into contact with specific substances. Meanwhile, dyshidrotic eczema is considered the blistering form of this condition and is often found on hands, palms, fingers or feet.
Causes of AD
Some causes of AD include scratching, sweat, heat, humid and dry weather, extremely cold weather, hot showers/baths, contact with chemicals such as cleaners, detergents, soap, contact with fabric, allergens such as dust or pollen, stress, contact with sand, smoke, or dirt, or even strenuous activities.
Common Symptoms of AD
Infants may experience dry, itchy skin or rash on the scalp or cheeks.
For children, scaly patches on the skin commonly found on the knees and elbows, thick skin, lightened or darkening skin spots, rashes on the neck, face, and around the eyes are common symptoms.
For adults, especially those who have experienced them as a child, can experience discolored skin in addition to the symptoms mentioned above.
If authorized, Adtralza® (tralokinumab) will be the first approved biologic treatment that specifically targets the IL-13 cytokine, a key driver of atopic dermatitis signs and symptoms.