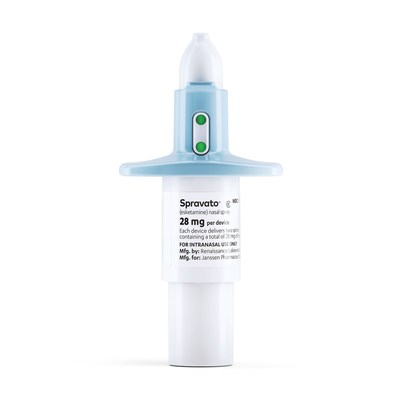
- SPRAVATO® is the first and only approved antidepressant medication shown to begin improving depressive symptoms with the first dose in this challenging to treat patient population - Approval is based on Phase 3 data showing SPRAVATO® reduced depressive symptoms in as little as four hours in some patients, with symptom improvement maintained through the four-week treatment period [03-August-2020]
|
TITUSVILLE, N.J., Aug. 3, 2020 /PRNewswire/ -- The Janssen Pharmaceutical Inc. Companies of Johnson & Johnson today announced that the U.S. Food and Drug Administration (FDA) has approved the supplemental new drug application (sNDA) for SPRAVATO® (esketamine) CIII nasal spray, taken with an oral antidepressant, to treat depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior.1 SPRAVATO® is the first and only approved medicine that has been shown to reduce depressive symptoms within 24 hours,1 providing a new option for significant symptom relief until a longer-term, comprehensive treatment plan can take effect. The effectiveness of SPRAVATO® in preventing suicide or in reducing suicidal ideation or behavior has not been demonstrated. Use of SPRAVATO® does not preclude the need for hospitalization if clinically warranted, even if patients experience improvement after an initial dose of SPRAVATO®. SPRAVATO® carries a Boxed Warning regarding a Risk Evaluation and Mitigation Strategy (REMS) and the risk of suicidal thoughts and behaviors. See below for Important Safety Information. SPRAVATO® will be made available at REMS certified treatment centers. Janssen is working to responsibly educate and certify treatment centers in accordance with the REMS so healthcare providers can offer SPRAVATO® to appropriate patients. Click to Tweet: #BREAKINGNEWS: FDA approves a new indication for @JanssenUS medicine for depressive symptom improvement in adults with major depression and suicidal behavior. Read full announcement here: https://ctt.ec/7yfjG+ Depression is the leading cause of disability worldwide and the condition most frequently associated with suicide.2 MDD is a serious disease that causes a significant, negative impact on the way people think, feel and act.3 Symptoms and severity vary by person and may include persistent feelings of sadness, hopelessness or tension; changes in sleep or appetite; difficulty concentrating or performing activities of daily living; lack of interest; and/or thoughts of harming themselves.3 "Many people who live with depression know all too well the feeling of desperation. If that major depression progresses to active suicidal thoughts, it's crushing, and they need options to help change the trajectory of their acute depressive episode," said Theresa Nguyen, Chief Program Officer, Mental Health America. "Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing." The sNDA approval is based on two identical Phase 3 clinical trials in which SPRAVATO® plus comprehensive standard of care demonstrated a significant, rapid reduction of depressive symptoms within 24 hours, with some patients starting to respond as early as four hours. SPRAVATO® plus comprehensive standard of care led to a 15.9 and 16.0 point decrease on the Montgomery-Åsberg Depression Rating Scale (MADRS), a tool used to assess severity of depressive symptoms, in the two trials at 24 hours after the first dose of study medication. This compared to a reduction of 12.0 and 12.2 points in the placebo plus comprehensive standard of care group. The comprehensive standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant and twice-weekly treatment visits for four weeks, during which patients received SPRAVATO® 84 mg or placebo nasal spray.4,5 Both the SPRAVATO® and placebo groups continued to improve between four hours and 25 days, with 41 percent and 43 percent of the SPRAVATO® plus comprehensive standard of care group achieving clinical remission of depression (minimal or no symptoms) compared with 34 percent and 27 percent in the placebo groups, by the end of the double-blind period, in the two trials, respectively.4,5 "It is astonishing to me that despite what we know about the risk of serious suicidal ideation in the context of major depression, patients with suicidal ideation have previously been excluded from nearly all studies examining antidepressant treatment efficacy. There is an immense need for high quality evidence showing effective and rapid antidepressant action in this population," said Gerard Sanacora,* Ph.D., M.D., Director, Yale Depression Research Program, Co-Director, Yale New Haven Hospital Interventional Psychiatry Service, and esketamine clinical trial investigator. "The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission." In the two Phase 3 trials, improvement in the severity of suicidality at 24 hours was measured using a standardized global scale. The treatment difference between the two groups was not statistically significant on this key secondary endpoint. Both SPRAVATO® and placebo in combination with comprehensive standard of care showed a similar reduction on this measure.4,5 The safety profile observed in the trials was consistent with previous studies of SPRAVATO® in treatment-resistant depression (TRD), adding to the established body of safety and efficacy evidence. The most common side effects included dissociation (feeling disconnected from yourself, your thoughts, feelings, space and time), dizziness, sedation (sleepiness), increased blood pressure, hypoesthesia, vomiting, euphoric mood and vertigo.1 With this new indication, SPRAVATO® can be prescribed to treat depressive symptoms in two MDD subpopulations of adults with high unmet need:
A full course of treatment for the new indication is twice weekly for four weeks, after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment. Please click here for the full Prescribing Information. "People living with major depression need more options to meet their most critical needs, and we're proud to help redefine how we treat ongoing and acutely worsening depressive symptoms," said Bill Martin, Global Therapeutic Area Head, Neuroscience, Janssen Research & Development, LLC. "SPRAVATO can now help patients with challenging to treat depression find significant and swift relief from debilitating depressive symptoms, offering those living with this serious mental health condition the possibility of a better future." Once SPRAVATO® is determined as an appropriate treatment option, in accordance with the REMS, patients will be treated at a certified treatment center trained to administer the medicine and address their needs. Janssen will educate healthcare providers and payers on this updated label to ensure appropriate patients are evaluated and the full course of treatment is delivered in a safe, appropriate and controlled manner for patients to receive the maximum treatment benefit. For patients who need help getting started on SPRAVATO® and staying on track, Janssen CarePath offers a comprehensive support program. Janssen CarePath provides information on insurance coverage, potential out-of-pocket costs and treatment support, and identifies options that may help make treatment more affordable, including the Janssen CarePath Savings Program for commercially insured patients who are eligible. Those who don't have commercial or private health insurance, or who aren't eligible for the Janssen CarePath Savings Program, can visit JanssenPrescriptionAssistance.com for more information about other resources that may help with their out-of-pocket medication costs. *Dr. Sanacora has received research support from Janssen and has served as a paid consultant to the company. About SPRAVATO® SPRAVATO® is approved in the United States, in conjunction with an oral antidepressant, to treat adults with treatment-resistant depression (TRD) and depressive symptoms in adults with MDD with acute suicidal ideation or behavior. SPRAVATO® has been submitted for health authorities' review for TRD and adults with MDD who have current suicidal ideation with intent in other markets around the world, including Europe. The FDA granted Breakthrough Therapy Designation to esketamine nasal spray for TRD in November 2013 and for MDD with imminent risk for suicide in August 2016. About the SPRAVATO® Risk Evaluation & Mitigation Strategy (REMS)
About the Phase 3 Studies4,5 The primary efficacy endpoint of the double-blind, randomized, placebo-controlled, multicenter studies was a reduction in depressive symptoms at 24 hours after the first dose, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). The MADRS scale is a tool used to assess severity of depressive symptoms, allowing clinicians to evaluate 10 symptoms on a six-point scale to produce a total score of up to 60 points. A secondary efficacy endpoint measured improvement in severity of suicidality as measured by the revised Clinical Global Impression of Severity of Suicidality (CGI-SS-r), a seven-point scale developed by clinical experts that is a measure of the severity of suicidality as judged by the clinician's global impression. About the Janssen Pharmaceutical Companies of Johnson & Johnson Learn more at www.janssen.com. Follow us at www.twitter.com/JanssenUS. Janssen Pharmaceuticals, Inc. is one of the Janssen Pharmaceutical Companies of Johnson & Johnson. IMPORTANT SAFETY INFORMATION
What is SPRAVATO® (esketamine) CIII nasal spray? SPRAVATO® is a prescription medicine, used along with an antidepressant taken by mouth to treat:
SPRAVATO® is not for use as a medicine to prevent or relieve pain (anesthetic). It is not known if SPRAVATO® is safe or effective as an anesthetic medicine. It is not known if SPRAVATO® is safe and effective for use in preventing suicide or in reducing suicidal thoughts or actions. SPRAVATO® is not for use in place of hospitalization if your healthcare provider determines that hospitalization is needed, even if improvement is experienced after the first dose of SPRAVATO®. It is not known if SPRAVATO® is safe and effective in children.
Do not take SPRAVATO® if you:
If you are not sure if you have any of the above conditions, talk to your healthcare provider before taking SPRAVATO®. Before you take SPRAVATO®, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Taking SPRAVATO® with certain medicine may cause side effects. Especially tell your healthcare provider if you take central nervous system (CNS) depressants, psychostimulants, or monoamine oxidase inhibitors (MAOIs) medicines. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. How will I take SPRAVATO®?
What should I avoid while taking SPRAVATO®? Do not drive, operate machinery, or do anything where you need to be completely alert after taking SPRAVATO®. Do not take part in these activities until the next day following a restful sleep. See "What is the most important information I should know about SPRAVATO®?" What are the possible side effects of SPRAVATO®? SPRAVATO® may cause serious side effects including:
The most common side effects of SPRAVATO® when used along with an antidepressant taken by mouth include:
If these common side effects occur, they usually happen right after taking SPRAVATO® and go away the same day. These are not all the possible side effects of SPRAVATO®. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. Please see full Prescribing Information, including Boxed WARNINGS, and Medication Guide for SPRAVATO® and discuss any questions you may have with your healthcare provider. ### Cautions Concerning Forward-Looking Statements ### REFERENCES 1 SPRAVATO® [Prescribing Information]. Titusville, N.J., Janssen Pharmaceuticals, Inc. July 2020. MEDIA CONTACT:
SOURCE Janssen Pharmaceutical Companies of Johnson & Johnson |