Indica Labs, the leading provider of computational pathology software and services, is excited to announce a CE-IVD Mark for HALO Prostate AI, a deep learning-based screening tool designed to assist pathologists in identifying and grading prostate cancer in core needle biopsies.
|
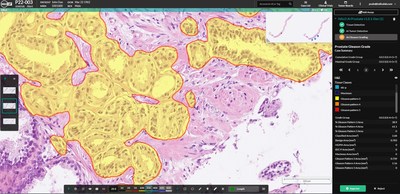
ALBUQUERQUE, N.M., May 23, 2022 /PRNewswire/ -- Indica Labs, the leading provider of computational pathology software and services, is excited to announce a CE-IVD Mark for HALO Prostate AI, a deep learning-based screening tool designed to assist pathologists in identifying and grading prostate cancer in core needle biopsies. Prostate cancer is the most common cancer diagnosed in men. With over 1.4 million cases reported worldwide in 2020, the incidence rates continue to rise with wider availability of screening tests, such as PSA. Each prostate case consists of multiple biopsy core whole slide images, which must be assessed by a pathologist for the presence of tumor and, if present, a Gleason score is reported. This represents a large workload for pathologists who screen multiple cases daily. HALO Prostate AI is designed to work alongside the pathologist to improve efficiency and to add a layer of quality control to ensure diagnostic accuracy. Developed in close collaboration with Dr. Yuri Tolkach and colleagues from the University of Cologne, HALO Prostate AI was trained using over 870,000 training patches obtained from the annotation of digital scans representing the full spectrum of prostate cancer subtypes and Gleason grades, as well as benign tissue. The algorithm achieved high sensitivity (95- 100%), specificity (88-98%) and negative predictive value (98- 100%) in validation studies performed on 4,973 core needle biopsies from three independent cohorts sourced from hospitals in Austria and Germany. Highlighting the unique benefit of HALO Prostate AI screening on diagnostic accuracy and patient care, the algorithm correctly detected tumor in 26 cores within the validation study that were originally reported as tumor negative. In a separate study, Gleason scores obtained from HALO Prostate AI were compared against scores assigned by pathologists located in ten globally distributed hospitals. Nine pathologists who participated in the study were board-certified in genitourinary pathology. High concordance was achieved for two separate cohorts with representative average quadratic Kappa scores of 0.8 and 0.7. "I am very excited about the digital future of pathology," commented Dr. Tolkach. "With tools such as HALO Prostate AI, our work can be substantially optimized while controlling for high-quality, reliable, and objective diagnostics. HALO Prostate AI showed very high accuracy in the large multi-institutional study for tumor detection and Gleason Grading in prostate biopsies. It's really enjoyable to work back-to-back with such a powerful AI assistant." Dr. Peter Caie, Principal Scientist of AI Collaboration at Indica Labs, added, "This has been a hugely positive collaboration with Dr. Tolkach and contributing pathologists from around the world. We set out to build a clinical-grade algorithm that could improve turn-around time and diagnostic accuracy for prostate cancer patients, and I believe that is exactly what we have achieved. We are thrilled to see the first of many AI algorithms from Indica Labs attain CE-IVD marking and to begin to be used in clinical settings to help alleviate the pressure of ever-increasing workloads." HALO Prostate AI is deployed through Indica Labs' CE-IVD marked HALO AP® platform to provide a fully validated and automated end-to-end workflow. HALO AP can operate as a fully functional, standalone case management system or can integrate with existing LIS solutions to allow outputs from HALO Prostate AI or other AI diagnostics to be accessible directly from the LIS. Designed to be scanner agnostic, HALO Prostate AI was trained using digital scans obtained from multiple scanning platforms and was clinically validated against the Hamamatsu NanoZoomer® S360 and Leica GT450 platforms. About Indica Labs, Inc. For more information, please visit https://indicalab.com or contact info@indicalab.com. Contact:
SOURCE Indica Labs |