Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced strongly positive interim results of the first 64 chronic deep vein thrombosis (“DVT”) patients enrolled in the ClotTriever Outcomes Registry (“CLOUT”).
IRVINE, Calif., Jan. 25, 2021 (GLOBE NEWSWIRE) -- Inari Medical Inc. (NASDAQ: NARI) (“Inari”) a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced strongly positive interim results of the first 64 chronic deep vein thrombosis (“DVT”) patients enrolled in the ClotTriever Outcomes Registry (“CLOUT”). The subanalysis, which focused on patients with an estimated clot age over six weeks, was presented virtually at LINC 2021, the Leipzig Interventional Course by principal investigator, Steven Abramowitz, MD, a vascular surgeon at MedStar Health in Washington, DC.
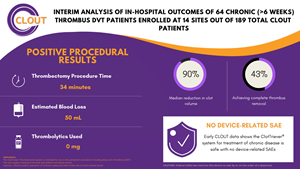
“Be it on-going COVID fears, delays in diagnosis or impediments to referral, many DVT patients have chronic disease by the time they finally meet a DVT interventionist. These physicians know that chronic clot is more wall-adherent and collagen-rich, rendering traditional lytic-based tools ineffective,” said Dr. Abramowitz. “The CLOUT chronic subanalysis has shown us that ClotTriever can offer these chronic DVT patients hope, removing a median 90% of their clot in a single session without the use of thrombolytics.” No serious device-related adverse events were reported across the 14 subanalysis sites, median blood loss was a modest 50cc and median thrombectomy procedure time was a short 34 minutes.
Also at LINC, Inari is hosting a live lunch symposium on Tuesday, January 26, at 12:30pm CET (6:30am ET), moderated by Dr. Gerard O’Sullivan, Interventional Radiologist, at Galway Clinic in Galway, Ireland including remarks on the latest Inari study experience by Dr. Abramowitz and Dr. Michael Jolly, Interventional Cardiologist, at OhioHealth in Columbus, OH.
Inari’s presence at LINC underscores the company’s developing commercialization efforts outside the United States. “With the recent CE Mark approval of our complete product portfolio, we look forward to providing more updates as our commercial expansion progresses,” said Bill Hoffman, Inari’s Chief Executive Officer. “Our focus on treating and transforming the lives of venous patients, now globally, remains unchanged. While we are pleased that we have treated over 20,000 VTE patients, we recognize that our work is only beginning, as there remain millions more in need of better treatment options around the world.”
About Inari Medical, Inc.
Inari Medical, Inc. is a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases. Inari has developed two minimally-invasive, novel catheter-based mechanical thrombectomy devices that are designed to remove large clots from large vessels and eliminate the need for thrombolytic drugs. The company purpose-built its products for the specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism, or VTE: deep vein thrombosis and pulmonary embolism. The ClotTriever system is 510(k)-cleared by the FDA and CE Mark approved for the treatment of deep vein thrombosis. The FlowTriever system is 510(k)-cleared by the FDA and CE Mark approved for the treatment of pulmonary embolism and clot in transit in the right atrium.
Investor Contact:
Westwicke Partners
Caroline Corner
Phone +1-415-202-5678
caroline.corner@westwicke.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e31f51c9-3aa1-44a0-aeed-235a7b796432