“Start-Up to Watch” in the June 8, 2018 issue of the MedTech Strategist.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20180622005088/en/
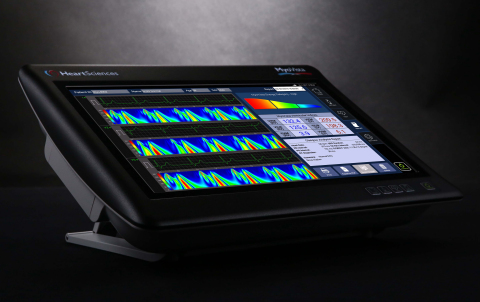
MyoVista Wavelet ECG Cardiac Testing Device (Photo: Business Wire)
“It is an honor to be recognized by MedTech Strategist as a Start-Up to Watch,” said Mark Hilz, CEO. HeartSciences is working to bridge today’s “diagnostic gap” in cardiac care by providing low-cost effective solutions that help to identify patients prior to experiencing an adverse cardiac event, such as a heart attack.
“The current screening paradigm for heart disease is missing people at early stages, when diseases are most treatable. HeartSciences has taken a ubiquitous screening tool, the 12-lead ECG, and has, through the application of signal processing and artificial intelligence, turned it into a powerful tool that can detect diastolic dysfunction, an early indicator of most cardiac diseases.”
--MedTech Strategist by Mary Stuart
About HeartSciences
HeartSciences is advancing the field of electrocardiography through the application of wavelet signal processing and artificial intelligence to develop next generation ECG technology. Wavelet signal processing is currently used in many different industries as an important tool to provide insights and new valuable data related to spectral analysis of a signal. HeartSciences’ MyoVista wavECG Cardiac Testing Device is a 12-lead resting electrocardiograph utilizing continuous wavelet transform (CWT) based signal processing. Patented informatics focus on energy related information rather than conventional voltage-based information. HeartSciences’ mission is to enable accurate, affordable screening for the early detection of heart disease.
In addition to the proprietary informatics, the MyoVista wavECG device also features the capabilities of a full featured 12-lead resting ECG including analysis using the Glasgow Algorithm, one of the world’s most respected interpretive algorithms. The device has a 15.6-inch high-resolution touchscreen display and incorporates many features commonly associated with a tablet device requiring minimal user training as well as easy and intuitive use. The MyoVista wavECG device is not currently approved for sale or distribution in the United States and is not currently FDA cleared.
HeartSciences is a privately-held U.S. corporation based in Southlake, Texas.
For more information visit www.heartsciences.com.
Media
The following link leads to a multimedia support platform designed to provide rapid download of media elements needed to develop your story about HeartSciences and MyoVista Technology.
https://digitalmedia.vnr1.com/2018/06/20/heartsciences/
View source version on businesswire.com: https://www.businesswire.com/news/home/20180622005088/en/
Contacts
HeartSciences
Gene Gephart, +1-737-346-1089
gene.gephart@heartsciences.com
or
Investor Contact
Zimmons International Communications
Jennifer K. Zimmons, +1-917-214-3514
jzimmons@zimmonsic.com
Source: HeartSciences