Geneoscopy Inc. , a life sciences company focused on the development of diagnostic tests for gastrointestinal health, announced today it submitted a Premarket Approval (PMA) application to the U.S. Food and Drug Administration.
|
|
| [24-January-2023] |
|
FDA's Decision Forthcoming for Innovative Test with Agency's Breakthrough Device Designation ST. LOUIS, Jan. 24, 2023 /PRNewswire/ -- Geneoscopy Inc., a life sciences company focused on the development of diagnostic tests for gastrointestinal health, announced today it submitted a Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA) for its noninvasive, stool-based, at-home screening test to detect colorectal cancer (CRC) and advanced adenomas (AA) in average-risk individuals. The FDA designated the test as a Breakthrough Device in January 2021. "The impact this test can have on patient health is beyond exciting, as is the validation of our stool RNA platform."
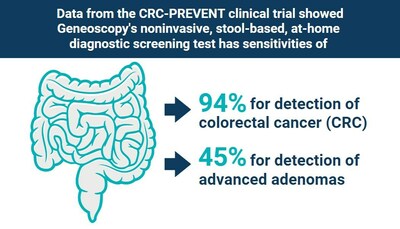
"Over 50 million Americans between the ages of 45 and 85 are eligible to be screened for CRC.1,2 Unfortunately, despite CRC being the second leading cause of cancer death in the U.S., millions of eligible Americans do not get screened – many due to a lack of knowledge of the importance of screening, lack of access to screening and concerns about the invasive nature of options like colonoscopy," said Dr. Erica Barnell, Chief Science Officer and Geneoscopy's co-founder. "We are committed to closing this gap for patients who require screening and look forward to working with the FDA to bring a convenient and reliable screening option to patients that may allow for earlier detection and treatment – and potentially save lives." The PMA submission is based on the favorable results from the pivotal CRC-PREVENT trial, in which Geneoscopy's test demonstrated 94% sensitivity for CRC and 45% sensitivity for advanced adenomas, representing the highest sensitivity profile reported to date for any noninvasive CRC screening test in a prospective clinical study. Additionally, in the 45-49 age population, Geneoscopy's test demonstrated 100% sensitivity for cancer and 44% sensitivity for advanced adenomas, at an 89% specificity. CRC-PREVENT is the first prospective study of a stool-based test to report CRC sensitivity in patients ages 45-49, an age group most recently added to the recommended screening population by the United States Preventive Services Task Force and the American Cancer Society. The robust sensitivity profile for CRC and advanced adenomas will offer significant value to this patient population. "The completion of our PMA submission is a major milestone – Geneoscopy's first regulatory approval application," said Andrew Barnell, Chief Executive Officer and Geneoscopy's co-founder. "This product and the years of effort from our team that have brought us here exemplify the dedication to our mission to empower patients and providers to transform gastrointestinal health through innovative diagnostics. The impact this test can have on patient health is beyond exciting, as is the validation of our stool RNA platform. We believe this platform will yield additional tests, including increasingly more precise CRC screening and high-value diagnostic testing for other gastrointestinal indications." About CRC-PREVENT CRC-PREVENT was a Phase 3 prospective, single-arm study designed to evaluate the efficacy of Geneoscopy's noninvasive, stool-based, at-home screening test to detect colorectal cancer and advanced adenomas in average-risk individuals. Using a collection kit, participants submitted self-collected stool samples via express delivery and underwent an optical colonoscopy examination. All significant lesions discovered during the colonoscopy were biopsied or removed and sent for histopathology. A comparative analysis was conducted to determine sensitivities and specificities for colorectal cancer, advanced adenomas, non-advanced adenomas, hyperplastic polyps, and colonoscopies with no findings. About Colorectal Cancer & Screening Responsible for over 50,000 deaths annually, colorectal cancer (CRC) is the second leading cause of cancer death in the United States. CRC usually begins as a growth (or polyp) that may or may not develop into cancer over time. Early detection and treatment are crucial to improve survival; however, many newly diagnosed patients suffer from advanced disease. Colonoscopy remains the gold standard for CRC screening in the U.S. Yet this method is frequently met with patient aversion due to its required bowel preparation, sedation, and potential time away from work. Currently available noninvasive screening methods demonstrate lower sensitivity to detect early-stage CRC and high-risk precancerous lesions, including advanced adenomas, which are estimated to be a precursor in 95 percent of CRC cases.3 About Geneoscopy Inc. Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Leveraging its proprietary, patented stool-derived eukaryotic RNA (seRNA) biomarker platform, Geneoscopy's mission is to empower patients and providers to transform gastrointestinal health through innovative diagnostics. Beyond colorectal cancer screening, Geneoscopy is developing tests for diagnosis, treatment selection, and therapy monitoring in other disease areas in partnership with leading universities and biopharmaceutical companies. For more information, visit www.geneoscopy.com and follow the company on LinkedIn. Geneoscopy Inc. Forward-Looking Statements The information in this release includes information about Geneoscopy's future plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas, which constitute forward-looking statements. These forward-looking statements are based on the Company's reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company's control. Geneoscopy's actual results and financial condition may differ materially from those indicated in the forward-looking statements. Although the Company believes that its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved, and the actual results may differ substantially from the projected results. Media Contact References
SOURCE Geneoscopy |