Genentech, Inc.
NEWS
Last month, biopharmas let go or projected they would let go of less than 500 people combined, based on BioSpace estimates, down almost 1,000 from January 2025. Still, competition for open jobs remains strong, with employed and unemployed biotech and pharma professionals eyeing their next roles.
Genentech, a member of the Roche Group, plans to open the facility in 2029 to ramp up capacity to make obesity candidates, including the dual GLP-1/GIP receptor agonist CT-388.
The agreement, which BMO Capital Markets called a “mild positive” for Structure, appears to address Roche’s concerns about the composition of investigational weight loss drug CT-996.
Roche CEO Thomas Schinecker said during the company’s Q3 call that it is “not done” with deals—a promise he delivered on with Tuesday’s solid tumor pact with AI-focused Caris Life Sciences.
Genentech is letting go of 118 employees in South San Francisco. The news comes about two months after the biotech ended a partnership with Adaptive Biotechnologies.
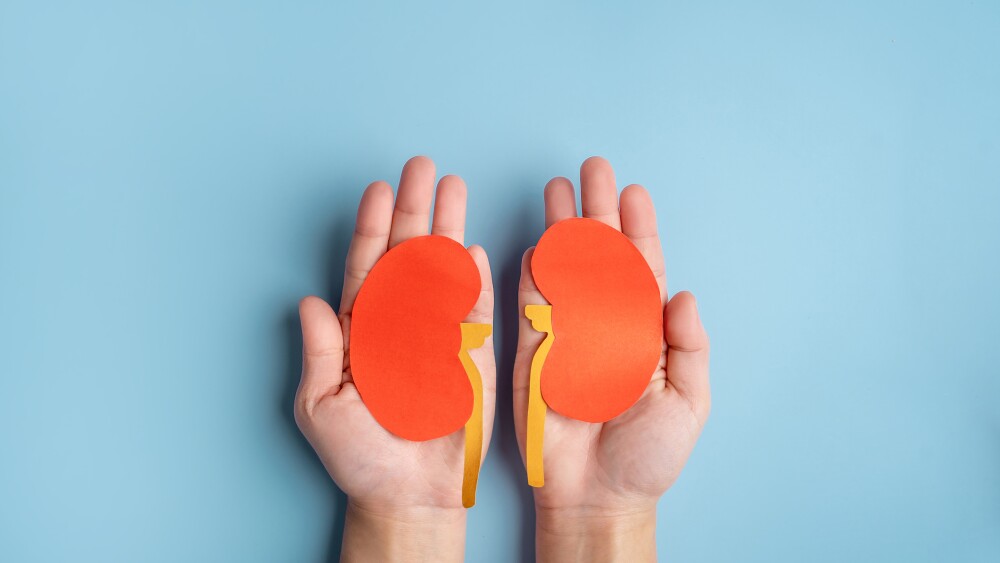
The anti-CD20 antibody, developed by Roche’s Genentech along with Biogen and already approved for multiple oncology indications, is branching into autoimmune disease. Its B cell–targeting mechanism of action gives it activity in improving kidney conditions in patients with lupus nephritis.
The latest round of terminations, which will take effect Sept. 15, comes after Genentech fired more than 500 employees in the last 15 months.
Roche and Genentech were unable to sufficiently demonstrate the benefit of using Columvi in an earlier treatment setting for DLBCL in a U.S. population, according to the FDA.
California’s life sciences manufacturing jobs dipped 3.7% in 2024, according to a new Biocom California report. Still, several companies made—and continuing making—significant manufacturing investments in the state as key trends shape the discipline.
JOBS
IN THE PRESS