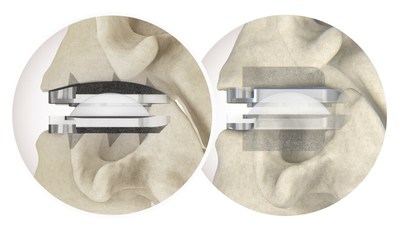
Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the completion of the 250th procedure in the United States with its newly FDA-approved cervical total disc replacement system of prodisc solutions.
WEST CHESTER, Pa., Dec. 14, 2022 /PRNewswire/ -- Centinel Spine Inc.®, ("the Company") a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the completion of the 250th procedure in the United States with its newly FDA-approved cervical total disc replacement system of prodisc solutions. This milestone, swiftly achieved since the Company announced the 100th completed procedure of prodisc C Vivo in the U.S. on October 27th and the first prodisc C SK on November 10th, further validates the value the comprehensive prodisc system offers surgeons to allow for better matching of the disc to the patient anatomy. Neurosurgeon John Edwards, MD, from Summit Brain, Spine and Orthopedics in Lehi, Utah, said, "I believe there is significant value in optionality with multiple discs, which provides the surgeon with the ability to meet a patient's particular needs. In the last decade, progress has been made in advancing disc arthroplasty and there is now more data on cervical total disc replacement than most other spine surgeries. The next step will be improving clinical outcomes through tailoring implant selection based on the patient." The prodisc C Vivo system has been in clinical use internationally since 2009 and is currently one of the most frequently implanted TDR devices in the world. The device has a streamlined surgical technique with keel-less fixation and combines a unique anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation. The prodisc C SK device features a flat endplate design for optimized implant positioning that allows surgeons to address individual patient anatomy—and a low-profile central keel that provides immediate fixation and enables a streamlined keel preparation technique. "A system of cervical total disc replacement options gives surgeons the advantage of selecting discs that best fit each patient and surgical challenge," noted Centinel Spine CEO Steve Murray. "The rapid achievement of today's milestone suggests that surgeons recognize this benefit and are embracing the ability to select the optimal device with prodisc C Vivo and prodisc C SK." Both the prodisc C Vivo and prodisc C SK incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.* * Data on file. About Centinel Spine, LLC Centinel Spine®, LLC is a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access. The company offers a continuum of trusted, brand-name, motion-preserving and fusion solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven technology platforms in the world for total disc replacement (prodisc®) and Integrated Interbody™ fusion (STALIF®). Centinel Spine continues to advance its pioneering culture and corporate mission to become a catalyst of change in the spine industry and alter the way spine surgery is perceived. Centinel Spine remains the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction. For more information, please visit the company's website at www.CentinelSpine.com or contact: Centinel Spine Media
SOURCE Centinel Spine, LLC |