Technavio has announced their latest drug pipeline analysis report on celiac therapeutics. The report includes a comprehensive research on the pipeline molecules under investigation by the pharmaceutical companies within the defined data collection period for the treatment of celiac.
LONDON--(BUSINESS WIRE)-- Technavio has announced their latest drug pipeline analysis report on celiac therapeutics. The report includes a comprehensive research on the pipeline molecules under investigation by the pharmaceutical companies within the defined data collection period for the treatment of celiac. The report also includes a study of the pipeline molecules in various stages including, on-going clinical trials, discovery, and pre-clinical.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20180910005697/en/
Technavio has published a new report on the drug development pipeline for the treatment of celiac therapeutics, including a detailed study of the pipeline molecules. (Graphic: Business Wire)
Celiac therapeutics: An overview
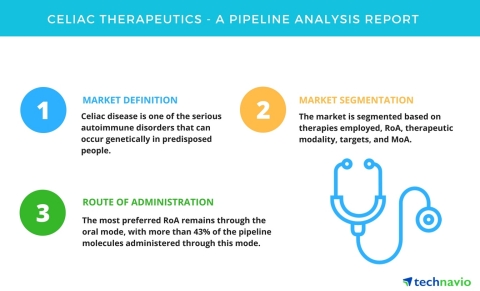
Celiac disease is also known as celiac sprue, non-tropical sprue, and gluten-sensitive enteropathy. It is one of the serious autoimmune disorders that can occur genetically in predisposed people. In this disease, the ingestion of gluten damages the inner wall of the small intestine. Celiac disease can occur in individuals of any age group at any time during their lifespan. It can develop into other critical health problems if left untreated. It can also lead to other autoimmune disorders such as multiple sclerosis, anemia, type I diabetes, infertility and miscarriage, short stature, dermatitis herpetiformis, and intestinal cancers.
Celiac disease affects children as well as adults across the world. As per National Institutes of Health (NIH), it has been found to be more common in Caucasians. In addition, it is being diagnosed more in females. It is estimated that one in 141 US citizens is affected by celiac disease. Furthermore, the occurrence of celiac disease is common in people who are affected by diseases, such as Down Syndrome, Turner Syndrome, and type 1 diabetes.
This report is available at a USD 1,000 discount for a limited time only: View market snapshot before purchasing
Celiac therapeutics: Segmentation of pipeline molecules
Technavio’s research segments the pipeline molecules based on different phases of drug development including, therapies employed, route of administration (RoA), mechanism of action (MoA), therapeutic modality, and the targets for the drugs under development. In the current drug pipeline, the most preferred RoA remains through the oral mode, with more than 43% of the pipeline molecules administered through this mode.
Between companies and institutions, companies led the drug development space for celiac therapeutics. Some of the key players include Amgen, Anyra Biotech, AnTolRx, BioLineRx, and Bioniz.
Looking for more information? Request your free sample today
Some of the key topics covered in the report include:
1. Scope of the Report
2. Regulatory Framework
3. Drug Development Landscape
- Drugs under development
4. Drug Development Strategies
- Therapies employed
- Route of administration
- Therapeutic modality
- Mechanism of action
5. Recruitment Strategies
- Geographical coverage
- Recruitment status
- Gender
- Age
6. Key Companies
- Type of players
- Company overview
7. Discontinued and Dormant Molecules
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 10,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
If you are interested in more information, please contact our media team at media@technavio.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20180910005697/en/
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
www.technavio.com
Source: Technavio Research







