Major Milestone Enables Clinical Development Program for Immuno-Oncology, Research on New Uses and Development of Therapeutic Analogs
Major Milestone Enables Clinical Development Program for Immuno-Oncology, Research on New Uses and Development of Therapeutic Analogs
DANVILLE, Calif.--(BUSINESS WIRE)-- BryoLogyx, Inc., today announced that it has completed the synthesis of bryostatin-1 molecule, pursuant to FDA’s Good Manufacturing Practice (GMP) regulations. Bryostatin-1 is the company’s lead compound being developed to improve patient outcomes by amplifying the response and increasing the durability of targeted cancer immunotherapies. The GMP synthesis, accomplished in partnership with Albany Molecular Research Inc. (AMRI), a global contract research, development and manufacturing organization (CDMO), paves the way for BryoLogyx’s planned clinical program with bryostatin-1 in immuno-oncology, additional research on the compound’s potential in other therapeutic areas, and the development of next generation synthetic analogs.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201013005116/en/
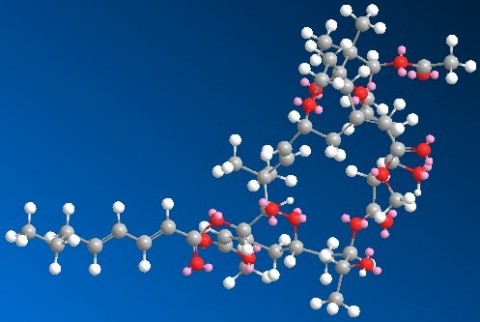
(Photo credit: BryoLogyx, Inc.) DANVILLE, CA (October 13)—BryoLogyx, Inc. announced today that it has chemically-synthesized the bryostatin-1 molecule (above) under FDA’s Good Manufacturing Practice (GMP) regulations. Its manufacture is in preparation for clinical trials to study its potential to improve the response to cancer immunotherapies and additional preclinical research. The molecule was originally sourced from a marine invertebrate organism by the NCI. Bryostatin-1 is a potent, highly complex macrolide that had proved nearly intractable to chemically synthesize. The manufacture was based on a fully synthetic process developed at Stanford University by Dr. Paul Wender and colleagues www.bryologyx.com.
The GMP-manufactured compound is based on the landmark patented synthesis process for bryostatin-1 reported in Science (2017) and developed by Paul Wender, PhD, and colleagues at Stanford University and licensed to BryoLogyx. Bryostatin-1 is an extremely complex molecule, originally isolated from a marine organism more than 50 years ago that has generated extensive research interest based on its protein kinase C (PKC) - modulating activity that affects many cellular processes. Much of the world’s supply to date was produced years ago by the National Cancer Institute (NCI), through a costly extraction from its marine source organism which is impractical for commercial development; that supply is largely depleted.
“The Wender synthesis process, which has been compared to the conquest of Mt. Everest, is a foundational pillar of BryoLogyx,” said Thomas Loarie, CEO of BryoLogyx. “The Wender method’s translation into a scalable and economical process to sustainably supply clinical grade bryostatin-1 is a key step towards defining the molecule’s potential in immuno-oncology through clinical trials and exploring additional therapeutic opportunities.” He noted that synthetic bryostatin-1 will be integrated into the company’s clinical program, which is expected to begin early next year.
“There exists an extensive body of clinical and preclinical research from the NCI and other laboratories that suggests that bryostatin-1 has a broad range of potential therapeutic applications. Its development as a therapeutic has been limited until today by supply. The availability of bryostatin-1 provides an important avenue for continued exploration of this molecule in cancer, auto-immune diseases, anti-inflammatory diseases, and infectious diseases,” said Dr. Wender, Bergstrom Professor of Chemistry at Stanford University; and cofounder and Board of Directors member at BryoLogyx.
BryoLogyx recently announced that the Company had entered into a Cooperative Research and Development Agreement (CRADA) with the National Cancer Institute (NCI) to conduct its first clinical trial with bryostatin-1 in patients with relapsing or refractory CD22 expressing acute lymphoblastic leukemia (ALL) and lymphoma. The study will evaluate bryostatin-1’s safety and tolerability, its ability to upregulate the CD22 antigen, an essential target of CD 22-directed antibody drug conjugates (ADCs) and CAR T cell therapies. Subsequent studies will evaluate bryostatin-1’s ability to upregulate target antigens in a variety of other B cell hematologic malignancies.
Underscoring the broad impact of this synthesis, earlier this year BryoLogyx announced an agreement with Neurotrope, to supply that company with synthetic bryostatin-1 for use in developing a treatment for Alzheimer’s disease and other neurological disorders.
Christopher Conway, President, AMRI, noted, “The GMP synthesis of this extraordinarily complex molecule took more than two years of work at our facilities in Hyderabad, India; Albany, NY, and Grafton, WI. The close collaboration on the project among our drug development team, BryoLogyx, and Dr. Wender, underscores how AMRI works with partners to produce complex pharmaceuticals and become integral to our partners’ supply chains.”
About BryoLogyx
BryoLogyx Inc. is advancing a new class of cancer immuno-therapeutics. The Company’s lead compound, bryostatin-1, is being developed as a cornerstone of cancer immunotherapies to amplify the immune response, increase treatment durability, and improve outcomes. BryoLogyx has exclusive rights from Stanford University to intellectual property covering the synthesis and methods of use of bryostatin-1 in cancer and other diseases. Learn more at www.bryologyx.com.
About AMRI
AMRI, a contract research development and manufacturing organization, partners with the pharmaceutical and biotechnology industries to improve patient outcomes and quality of life. AMRI’s team combines scientific expertise and market-leading technology to provide a complete suite of solutions in discovery, development, analytical services, and API and drug product manufacturing. www.amriglobal.com
View source version on businesswire.com: https://www.businesswire.com/news/home/20201013005116/en/
Contacts
Thomas M. Loarie
CEO, BryoLogyx
tloarie@bryologyx.com
Peter Steinerman
Steinerman Biomedical
prsteinerman@gmail.com
Source: BryoLogyx, Inc.
Smart Multimedia Gallery
(Photo credit: BryoLogyx, Inc.) DANVILLE, CA (October 13)—BryoLogyx, Inc. announced today that it has chemically-synthesized the bryostatin-1 molecule (above) under FDA’s Good Manufacturing Practice (GMP) regulations. Its manufacture is in preparation for clinical trials to study its potential to improve the response to cancer immunotherapies and additional preclinical research. The molecule was originally sourced from a marine invertebrate organism by the NCI. Bryostatin-1 is a potent, highly complex macrolide that had proved nearly intractable to chemically synthesize. The manufacture was based on a fully synthetic process developed at Stanford University by Dr. Paul Wender and colleagues www.bryologyx.com.







