Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), today announced that an abstract containing preclinical data for BMF-219 has been published in Blood, the journal of the American Society of Hematology.
- BMF-219 displays global disruption of menin, highlighting ‘219’s novel mechanism of action
- Molecule elicits broad impact on the complexes surrounding menin, resulting in strong modulation of MYC expression, highlighting potential in multiple cancer types
- BMF-219 demonstrated potent preclinical efficacy in diffuse large B cell lymphoma (DLBCL) cell lines as a single agent
REDWOOD CITY, Calif., Nov. 04, 2021 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel irreversible small molecules to treat and improve the lives of patients with genetically defined cancers, today announced that an abstract containing preclinical data for BMF-219 has been published in Blood, the journal of the American Society of Hematology.
The study, as published in Blood, analyzed how Biomea’s irreversible menin inhibitor, BMF-219, is impacting acute myeloid leukemia cells against the background of transcription factors in the GEO dataset. Menin is involved in many protein-protein interactions as part of a larger complex, with Menin and MLL/KMT2A being an example of one of those interactions. Here we investigated how ‘219 disrupts menin globally (selective disruption of global menin co-factors in addition to KMT2A).
Figure 1: Menin and various co-factors
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/210950de-0c6c-4204-9c99-a7ab3eb4a54c
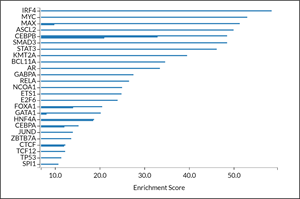
The study demonstrates further the ability of BMF-219 to modulate MYC expression in leukemia cells, offering the foundation for exploring its activity in DLBCL cells. Transcription factor (TF) activity inference was calculated by analysis of TF-binding sites established by chromatin immunoprecipitation sequencing (ChIP-seq) GEO datasets that overlap with BMF-219 mediated differentially expressed genes in MOLM-13 cells using a published statistical framework and algorithm. This analysis revealed MYC, and its co-factor MAX, as top TFs regulating this subset of differentially expressed genes by BMF-219, as TF activity inference was highly enriched for both proteins. Established menin co-factors (KMT2A, JUND) also emerged as top candidates in this dataset. These results strongly point toward altered MYC-activity mediated by BMF-219 in leukemia cells, prompting additional exploration in MYC-dependent lymphoid malignancies.
Figure 2: Transcription factor (TF) activity inference using ChIP-seq of differentially expressed genes in MOLM-13 cells incubated with 500 nM BMF-219 at 24 hours. Each bar represents a study in the GEO repository using the specified TF antibody. TFs with more than one bar represent multiple study sets in GEO that overlap with BMF-219 mediated differentially expressed genes. MYC and MAX are top TFs regulating this subset of differentially expressed genes (p=10-49.5). Established menin co-factors (KMT2A, JUND) also emerged as top candidates in this dataset.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/982b0dd0-91ce-482b-bd8a-fa06e9c01dd9
Using these results as the basis for further investigation, we explored BMF-219 and menin reversible inhibitors’ impact on cell viability in two different DLBCL double hit lymphoma (DHL) cell lines (DB and Toledo). Single-agent BMF-219 reduced >90% of cell viability in DB and Toledo cells, at 1.0μM and 0.36 μM, respectively. The IC50 values of BMF-219 were calculated near 0.3 mM for both DB and Toledo cells; however, the two reversible menin inhibitors tested were significantly less effective. One of the reversible inhibitors exhibited IC50 values at multi-fold higher drug concentrations than BMF-219 in both cell lines tested and the other reversible compound tested did not show sensitivity to either cell line.
“With this study, we gained valuable understanding and expanded our knowledge into the mechanism of action of our irreversible menin inhibitor, BMF-219. Besides its targeted selectivity profile, we also recognize the broad impact BMF-219 has on the complexes surrounding menin,” said Alex Cacovean, M.D., Biomea Fusion’s Executive Medical Director. “At low dosage levels, by targeting menin, we were able to inhibit MYC gene expression and demonstrated potent cell killing in two very aggressive cell lines, representative of underserved DLBCL subpopulations. We are very excited about these preclinical findings and we are hopeful to now clinically validate the effects of our irreversible menin inhibitor in MYC-positive, double expressor, and double hit DLBCL patients.”
Abstract Details
Title: Novel Irreversible Menin Inhibitor, BMF-219, Shows Potent Single Agent Activity in Clinically Relevant DLBCL Cells
Abstract Number: 148045
About Diffuse Large B Cell Lymphoma (DLBCL)
Diffuse large B cell lymphoma (DLBCL) is the most common subtype of Non-Hodgkin Lymphoma. DLBCL starts in white blood cells called lymphocytes and grows in lymph nodes. Every year, there are ~18,000 people in the United States that are diagnosed with DLBCL. Following initial treatment with standard chemotherapy, ~70% of people have a complete response and ~50% of patients are cured. For patients with relapsed or refractory DLBCL, median overall survival is between 6-7 months. Double Hit Lymphomas (DHL) and Double Expressor Lymphomas (DEL) are high grade B cell lymphomas (HGBLs) that have high MYC and BCL2 dependency.
About BMF-219
BMF-219 is an irreversibly binding inhibitor of menin, a protein that is known to play an essential role in oncogenic signaling in genetically defined leukemias. Preclinically, BMF-219 has demonstrated robust downregulation of key leukemogenic genes in addition to menin itself (via MEN1) in well-established MLLr AML cell lines. Additionally, BMF-219 has shown efficacy in multiple in vivo and in vitro models of acute leukemias. BMF-219 will be evaluated in a first-in-human trial in patients with relapsed or refractory acute leukemia with MLL/KMT2A gene rearrangement or NPM1 mutation.
About Biomea Fusion
Biomea Fusion is a biopharmaceutical company focused on the discovery and development of irreversible small molecules to treat patients with genetically defined cancers. An irreversible small molecule is a synthetic compound that forms a permanent bond to its target protein and offers a number of potential advantages over conventional reversible drugs, including greater target selectivity, lower drug exposure, and the ability to drive a deeper, more durable response. The company is utilizing its proprietary FUSION™ discovery platform to advance a pipeline of irreversible treatments against key oncogenic drivers of cancer. Biomea Fusion’s goal is to utilize its capabilities and platform to become a leader in developing irreversible small molecules in order to maximize the depth and durability of clinical benefit when treating various cancers.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding the potential safety, efficacy, and continued development of BMF-219, the timing for initiating clinical development, the timing of pre-clinical and clinical data announcement, the timing of nominating additional product candidates, the building out of our proprietary irreversible platform and progress made in early-stage small pipeline molecules through their preclinical development, including the timing for nominating development candidates in each program. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may,” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. Any forward-looking statements in this statement are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: the success, cost, and timing of the Company’s product candidate development activities and planned IND-enabling and clinical trials, the Company’s ability to execute on its strategy, regulatory developments in the United States, the Company’s ability to fund operations, and the impact that the current COVID-19 pandemic will have on the Company’s clinical trials and pre-clinical studies, supply chain, and operations, as well as those risks and uncertainties set forth in the Company’s filings with the United States Securities and Exchange Commission. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All forward-looking statements contained in this press release speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Contact:
Van Sandwick
Director, Investor Relations & Corporate Development
vsandwick@biomeafusion.com
(650) 460-7759

Figure 1
Menin and various co-factors
Figure 2