-- Biohaven plans to study intranasal vazegepant, a third generation, high affinity, selective and structurally unique, small molecule CGRP receptor antagonist, in pulmonary complications of COVID-19 disease -- Phase 2 study to start within weeks, in collaboration with Thomas Jefferson University and other academic medical institutions -- The clinical trial will assess the potential benefits of CGRP receptor-blockade in mitigating an excessive immune response which in some ca
|
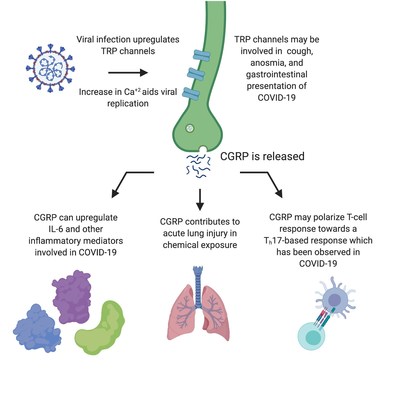
NEW HAVEN, Conn., April 9, 2020 /PRNewswire/ --Biohaven Pharma Holding Company Ltd. (NYSE: BHVN) ("Biohaven") today announced that it submitted Pre-IND/IND material to the U.S. Food and Drug Administration (FDA) to initiate a Phase 2 study of vazegepant, an intranasal, high-affinity calcitonin gene-related peptide (CGRP) receptor antagonist, for the treatment of COVID-19 infection associated pulmonary complications. The IND was approved by the Division of Pulmonary, Allergy, and Critical Care at FDA on April 8th and Biohaven was informed that the study may proceed immediately. Vazegepant is currently advancing to Phase 3 development for the acute treatment of migraine in adults under the Division of Neurology, having recently reported a successful end of Phase 2 clinical and nonclinical interaction with FDA. Vlad Coric, M.D., Chief Executive Officer of Biohaven commented, "Given the unprecedented global threat to life that COVID-19 infection represents, there is an urgent need to test novel approaches to mitigating the consequences of the infection, including reducing the hyper-immune reaction that drives much of the morbidity and mortality in the pulmonary effects of this viral disease." Dr. Coric added, "This pandemic is a call to action for our entire industry. I am grateful to our collaborators at the pulmonary institute at Thomas Jefferson and the team at Biohaven that moved so quickly to develop a clinical protocol and submit this IND to the FDA in the weeks after the pandemic first developed. We owe a debt of gratitude to the scientists at the NIH, FDA, academic centers and pharmaceutical companies who are working hard to study therapies that might battle this terrible disease -- the innovative efforts of the true unsung scientific heroes of this crisis will only be fully recognized once better treatments are developed to treat COVID-19." Jesse Roman, M.D., Professor, Jane & Leonard Korman Respiratory Institute - Jefferson Health and National Jewish Health, Thomas Jefferson University, and co-Principal Investigator in the proposed COVID-19 study commented, "Hospitals and our health care system are being overwhelmed by those patients who are progressing to serious COVID-19 infection. There is an urgent need to identify treatments that can mitigate the inflammatory process in these patients and help stabilize our response to the pandemic. It's critical that we move quickly to test immune-modulating drugs like vazegepant and other agents as to assess if they can provide some benefit." Biohaven's proposed clinical study of vazegepant is a Phase 2 double blind, randomized, placebo-controlled, safety and efficacy trial of intranasal vazegepant for COVID-19 infected hospitalized patients requiring supplemental oxygen. Data from the proposed study will allow characterization of the relative safety and efficacy of intranasal vazegpant versus placebo in the treatment of COVID-19 leading to hospitalization and the primary outcome measures will include pulmonary resolution of symptoms, or progression to ventilator support or death. The study will be overseen at Biohaven by Steven Schnittman, M.D., a seasoned infectious disease clinical researcher and pharmaceutical drug developer. Michael Baram, M.D., Thomas Jefferson University, Pulmonary Critical Care and Emergency Medicine, will be the Principal Investigator for the study. Donnie McGrath, M.D., Chief of Corporate Strategy of Biohaven commented, "There is a solid scientific rationale supporting testing of small molecule CGRP-antagonists as a way to mitigate the neuro-immune consequences of respiratory viral infections like COVID-19. While the biology is complex and CGRP has pleotropic effects on the immune system, CGRP release as a result of viral activation of TRP channels is implicated in COVID-19 features such as cough, fever and pain, and the subsequent release of interleukin 6 (IL-6). IL-6 is an important mediator of inflammation that is elevated in the sickest of COVID-19 patients. In addition, CGRP may be involved in the polarization of the T cell response in some COVID-19 patients towards the pro-inflammatory state characterized by Th17 and IL-17 (see Figure)." Acute lung injury is known to induce upregulation of transient receptor potential (TRP) channels which activates CGRP release contributing to acute lung injury (pulmonary edema with acute phase cytokine/mediator release, with immunologic milieu shift toward TH17 cytokines) followed by chronic lung injury with hyaline membrane formation, fibrosis and reduced diffusion capacity. Acute respiratory distress syndrome (ARDS), which is a common pathway resulting from diverse types of lung injury, is part of the pathogenic process that can occur after viral infections (see Figure). Because COVID-19 (SARS2) infection leads to an acute insult of pulmonary epithelia, it is hypothesized that a CGRP receptor antagonist may potentially blunt the severe inflammatory response at the alveolar level, delaying or reversing the path towards oxygen desaturation, ARDS, requirement for supplemental oxygenation, artificial ventilation or death. Biohaven is also involved in COVID-19 antiviral therapy development through its investment and clinical operations support that it provides to Kleo Pharmaceuticals, Inc. Kleo Pharmaceuticals is an immune-focused, biotech company with a platform of small molecules that is working to create "synthetic vaccines" to redirect the patient's own immune cells to attack viruses. Kleo Pharmaceuticals recently announced that it has entered into a research collaboration with South Korea-based Green Cross LabCell (GCLC), a pioneer in the next generation of allogeneic, or "off-the-shelf" natural killer (NK) cell therapies, to rapidly advance testing of both advanced technology platforms in combination as a potential therapy for COVID-19 patients. About COVID-19 About Vazegepant About Kleo Pharmaceuticals, Inc. About Biohaven Forward-looking Statement Biohaven Contact Kleo Contact NURTEC is a trademark of Biohaven Pharmaceutical Holding Company Ltd. ARMs, MATEs and SyAMs are trademarks of Kleo Pharmaceuticals, Inc.
SOURCE Biohaven Pharmaceutical Holding Company Ltd. |
||
Company Codes: NYSE:BHVN, OTC-PINK:PPTDF, Tokyo:4587, Tokyo:PPTDF |