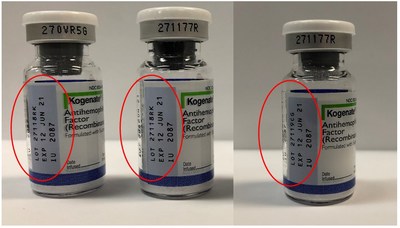
Bayer is voluntarily recalling two lots of Kogenate® FS antihemophilic factor (recombinant) 2000 IU vials in the United States to the patient level.
|
WHIPPANY, N.J., July 19, 2019 /PRNewswire/ -- Bayer is voluntarily recalling two lots of Kogenate® FS antihemophilic factor (recombinant) 2000 IU vials in the United States to the patient level. Certain vials from these two lots that were labeled as Kogenate FS actually contain the FVIII hemophilia A treatment, Jivi® antihemophilic factor (recombinant) PEGylated-aucl 3000 IU. The U.S. is the only country where affected products were distributed. We are working closely with the U.S. Food and Drug Administration to manage the recall and to minimize disruption to supply and inconvenience to patients. The affected lots, distributed from February 5, 2019 to July 15, 2019 from Bayer's distribution sites in Berkeley, CA and Shawnee, KS, are listed below:
While the majority of the mislabeled vials in the affected lots were recovered, approximately 990 of these vials were released in the U.S. The associated Jivi batch was expired as of August 2018. However, all stability specifications of this expired Jivi batch had continued to be met, as of April 2019. We have voluntarily recalled both lots in the interest of patient safety, and to ensure that any potentially impacted product is removed from pharmacy shelves, and that patients and their healthcare providers are alerted. Importantly, vials of Kogenate FS that are not associated with the affected lot numbers (27118RK and 27119CG) are not impacted and can continue to be used without interruption. There are no lots of Jivi or Kovaltry® antihemophilic factor (recombinant) product affected by this recall. Patient safety is Bayer's highest priority. We are carefully monitoring for any complaints or adverse event reports that may be related to this recall. The company is issuing this notification after discovering the issue earlier this week. Following an immediate internal assessment, Bayer contacted the FDA to inform the agency of the situation. Kogenate FS and Jivi are both medicines used to replace clotting factor (factor VIII or antihemophilic factor) that is missing in people with hemophilia A. Kogenate FS is approved to treat or control bleeding in adults and children with hemophilia A. Jivi is approved to treat and control bleeding in previously treated adults and adolescents (12 years of age and older) with hemophilia A. Patients in possession of vials from the affected lot numbers should immediately stop using the product and contact their physician. In addition, patients should contact their pharmacy to return the affected product. Bayer is notifying its distributors in writing to check their stock immediately and to discontinue the distribution and use of any affected product. For distributors with questions regarding the recall process, please contact the Bayer Recall Coordinator, Inmar, at 855-707-7518. Bayer is committed to providing our patients safe and effective therapy. We encourage healthcare providers and patients with questions to call our medical communications hotline with questions: 1-888-84-BAYER (1-888-842-2937). We understand that this news may be concerning for patients with hemophilia A who depend on these medicines. Bayer is committed to providing the most up-to-date and accurate information to those who may be affected by this issue. Bayer takes its 30-year partnership with the hemophilia community very seriously, and we remain deeply dedicated to meeting the needs of patients living with this life-long disease. Patients and healthcare providers are encouraged to report side effects or quality complaints of products to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer at http://adversereactions.bayer.com. Product Photos:
https://mma.prnewswire.com/media/949625/Product_Photos.jpg Kogenate® FS antihemophilic factor (recombinant) Indications and Important Safety Information Indications Kogenate® FS Antihemophilic Factor (Recombinant) is a medicine used to replace clotting factor (factor VIII or antihemophilic factor) that is missing in people with hemophilia A. Kogenate FS is used to treat and control bleeding in adults and children with hemophilia A. Your healthcare provider may give you Kogenate FS when you have surgery. Kogenate FS can reduce the number of bleeding episodes in adults and children when used regularly (prophylaxis). Kogenate FS can reduce the risk of joint damage in children without pre-existing joint damage when used regularly. Kogenate FS is not used to treat von Willebrand disease. Important Safety Information You should not use Kogenate FS if you are allergic to rodents (like mice and hamsters) or are allergic to any ingredients in Kogenate FS. Tell your healthcare provider if you have been told you have heart disease or are at risk for heart disease. You could have an allergic reaction to Kogenate FS. Call your healthcare provider right away and stop treatment if you get rash or hives, itching, tightness of the chest or throat, difficulty breathing, light-headed, dizziness, nausea or a decrease in blood pressure. Your body can make antibodies, called "inhibitors," against Kogenate FS, which may stop Kogenate FS from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to factor VIII. Other common side effects of Kogenate FS are local injection site reactions (pain, swelling, irritation at infusion site) and infections from implanted injection device. Tell your healthcare provider about any side effect that bothers you or does not go away. Call your healthcare provider right away if bleeding is not controlled after using Kogenate FS. For important risk and use information, please see full prescribing information. Jivi® antihemophilic factor (recombinant) PEGylated-aucl Indications and Important Safety Information INDICATIONS
IMPORTANT SAFETY INFORMATION
For additional important risk and use information, please see full Prescribing Information. KOVALTRY® antihemophilic factor (recombinant) Indications and Important Safety Information INDICATIONS
KOVALTRY® Important Safety Information
For important risk and use information, please see the full prescribing information. About Bayer Bayer is a global enterprise with core competencies in the life science fields of health care and nutrition. Its products and services are designed to benefit people by supporting efforts to overcome the major challenges presented by a growing and aging global population. At the same time, the Group aims to increase its earning power and create value through innovation and growth. Bayer is committed to the principles of sustainable development, and the Bayer brand stands for trust, reliability and quality throughout the world. In fiscal 2018, the Group employed around 117,000 people and had sales of 39.6 billion euros. Capital expenditures amounted to 2.6 billion euros, R&D expenses to 5.2 billion euros. For more information, go to www.bayer.us. Contact: Media Contact: Forward-Looking Statements This release may contain forward-looking statements based on current assumptions and forecasts made by Bayer management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer's public reports which are available on the Bayer website at www.bayer.com. The company assumes no liability whatsoever to update these forward-looking statements or to conform them to future events or developments.
SOURCE Bayer |