For the first quarter of 2019, BALT has announced a 34% rise in revenue as compared to the same period in 2018.
MONTMORENCY, France--(BUSINESS WIRE)-- For the first quarter of 2019, BALT has announced a 34% rise in revenue as compared to the same period in 2018. As a reminder, the company ended the 2018 financial year with revenues of €100 million. As for the last quarter of 2018, BALT achieved its highest level of operating income since the company was created.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190610005767/en/
(Graphic: Business Wire)
"We have had a wonderful start to 2019, with first quarter results fully in line with our goals," said Pascal Girin, BALT’s Chief Executive Officer. "The neurovascular sector is continuing to grow rapidly. We have seen very strong growth in our strategic markets (Europe, the US and China) mainly due to the profits generated through our major recent investments in these regions."
"2018 marked an important milestone in BALT's history with the global launch of new flagship products such as the Silk Vista Baby flow diverter, Catchview reperfusion stents, and the Optima coil,” said Pascal Girin. “Since 2018, our efforts have focused on two core projects: first, the implementation of a program to modernize our production lines and increase our capacity, and second, the extension of our sales coverage by establishing ourselves directly in certain countries. Based on success in both of these key initiatives, we are starting 2019 on solid ground. We are seeing strong market demand for our products as they become more widely available, and the rising number of orders from hospitals in recent months is evidence of this."
"In order to take full advantage of market potential, I recently solicited the shareholders – the Bridgepoint investment fund, the founding family and the management team – to support our growth programs through a capital increase. I am delighted that this initiative was approved unanimously at the last general assembly. I would like to thank the shareholders, just as I would like to thank all of BALT’s employees for their continued commitment, the commitment that enables us to provide patients over the world with the finest neurovascular products available."
"These excellent first-quarter results should be reinforced in the future thanks to regulatory clearances by the FDA or CE markings on eight of our products (flow diverters, stents, catheters, coils, embolic glues, etc.) over the past twelve months," added Mr. Girin. "Additionally, we plan to reinforce growth-generating investments in clinical studies, research and development, and production, which should accelerate our future growth.”
The company is ambitious to continue to meet the very strong market demand and to pursue its development in Europe and throughout the world while capitalizing on its existing strengths – its pioneering spirit and capacity for innovation in particular.
"BALT is a key player in the cutting-edge and highly promising interventional neuroradiology market. Thanks to an unparalleled range of products with superior technical performance, the company is now able to accelerate its growth, increase its production capacity, expand its distribution network and win new customers, all while preserving its unique expertise," said Pascal Girin. "For the rest of the year, we expect continued growth in our strategic regions. These will contribute considerably to the improvement of our results in 2019," he added.
BALT also expects its business activity in certain emerging countries to accelerate, particularly in Brazil, where the company enjoys a solid market position. The opening of offices in the country could be implemented sometime this year.
In view of these prospects and the current trends in the neurovascular market, the company confirms its objectives for 2019, with strong growth in revenue and operating income.
Key points:
- Growth in revenue of 34% in the first quarter of 2019 as compared to the same period in 2018, with total revenue of €100 million for 2018.
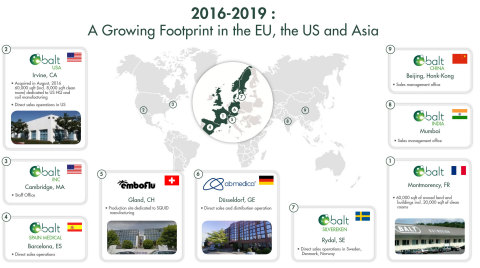
- Strong market penetration of certain BALT products following the opening of a manufacturing site in the United States, as well as offices in Europe and Asia.
- Introduction and implementation of a production capacity expansion program resulting in increased production volumes for BALT’s main industrial site by 30% in 2018. The objective is to accelerate this program in 2019.
- Obtaining US or European regulatory clearances for 8 products over the last 12 months.
View source version on businesswire.com: https://www.businesswire.com/news/home/20190610005767/en/
Contacts
Ryan Solomon (US): 949.788.1443 – ryan.solomon@balt-usa.com
Hanane Imbert (France): +33 (0)623461554 - Hanane.aouad@myeditors.fr
Source: BALT






