SAN DIEGO, Aug. 8, 2016 /PRNewswire/ -- Pfenex Inc. (NYSE MKT: PFNX) announced today positive immunogenicity and safety data from the Day 70 analysis of the Px563L anthrax vaccine study. The results demonstrate tolerable safety and significant immunogenicity after only 2 doses of Px563L.
"The Px563L data from the Day 70 analysis of the phase 1 study are very encouraging, with the vaccine being well-tolerated and affording potentially superior protection after only 2 doses," noted Dr. Hubert Chen, chief medical officer of Pfenex. Bertrand C. Liang, chief executive officer added, "These positive anthrax vaccine findings are a strong validation of our close partnership with BARDA, and Pfenex is grateful for the support of the United States government. We look forward to continuing the collaborative development of Px563L with BARDA and progressing towards a potential procurement of our next-generation anthrax vaccine to satisfy Strategic National Stockpile needs. Given our highly efficient production process, which leverages our Pfenex Expression Technology® platform, we believe we could supply the United States government's anthrax vaccine stockpile goal quickly and very cost efficiently, meeting a key articulated need of the United States government."
Study Results
The randomized, double-blind, placebo-controlled phase 1a study enrolled 3 cohorts (10 mcg, 50 mcg and 80 mcg) in a dose-escalating manner. Within each cohort, subjects received Px563L, RPA563 or placebo in an 8:8:2 ratio. Subjects were administered 2 doses of vaccine or placebo 28 days apart. The current analysis covers safety and immunogenicity assessment, including toxin-neutralizing antibody (TNA) expressed as 50% neutralizing factor (NF50), through Day 70. TNA NF50 threshold value > 0.56 has been correlated with significant survival after anthrax exposure in animal models.
After administration of 2 doses, 100% of Px563L subjects achieved a TNA NF50> 0.56 at Day 70 for both the 10 mcg and 80 mcg dose cohorts, and 87.5% achieved the target threshold in the 50 mcg dose cohort (Table 1 and Figure 1). For comparison, after subcutaneous (SC) administration of 3 Biothrax® doses (currently approved anthrax vaccine), 57.9% of subjects achieved a TNA NF50>0.56 at Day 70 (Vaccine 2014; 32:2217-2224).
An additional criterion for assessing anthrax vaccine immunogenicity success is for the lower confidence limit (LCL), or the lower bound of the two-sided 95% confidence interval for the percentage of subjects who met or exceeded the TNA threshold (TNA NF50> 0.56), to be greater than or equal to 40%.
All doses of Px563L were above the LCL at Day 70 (Figure 1 and Table 1), having demonstrated this activity after only 2 doses (10 mcg: 74%, 50 mcg: 55%, 80 mcg 74%). For comparison, Biothrax reported a LCL of 50.4% at Day 70 (Vaccine 2014; 32:2217-2224) following a three-dose regimen (Table 1).
Only mild Grade 1 injection site reactions were observed at the 10 mcg and 50 mcg Px563L doses as well as for all RPA563 doses. At the 80 mcg Px563L dose, in addition to Grade 1 findings, there were two Grade 2 reports of erythema (redness). There were no other Grade 2 or higher injection site reactions, specifically injection site pain, arm motion limitation, tenderness or swelling.
Table 1: Positive Day 70 Immunogenicity Results for Px563L
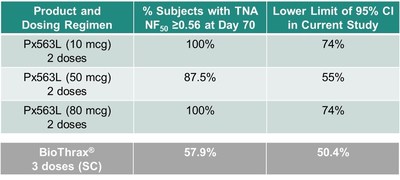
* BioThrax® data obtained from Vaccine 2014; 32:2217-2224
Photo - http://photos.prnewswire.com/prnh/20160806/396199
Figure 1: TNA NF50 Immunogenicity Response for Px563L, RPA563 and placebo (logarithmic scale)
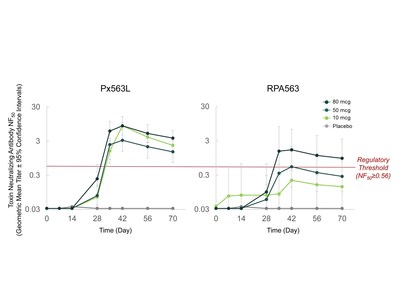
Photo - http://photos.prnewswire.com/prnh/20160806/396200
Cautionary Note Regarding Forward-Looking Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or Pfenex's future financial or operating performance. In some cases, you can identify forward-looking statements because they contain words such as "may," "will," "should," "expects," "plans," "anticipates," "could," "intends," "target," "projects," "contemplates," "believes," "estimates," "predicts," "potential" or "continue" or the negative of these words or other similar terms or expressions that concern Pfenex's expectations, strategy, plans or intentions. Forward-looking statements in this press release include, but are not limited to, statements regarding the future potential of Pfenex's product candidate Px563L and Pfenex's ability to supply the United States government's anthrax vaccine stockpile goal quickly and very cost efficiently. Pfenex's expectations and beliefs regarding these matters may not materialize, and actual results in future periods are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Actual results may differ materially from those indicated by these forward-looking statements as a result of the uncertainties inherent in the clinical drug development process, including, without limitation, Pfenex's ability to successfully demonstrate the efficacy and safety of its product candidates; the pre-clinical and clinical results for its product candidates, which may not support further development of product candidates or may require Pfenex to conduct additional clinical trials or modify ongoing clinical trials or regulatory pathways; challenges related to commencement, patient enrollment, completion, and analysis of clinical trials; Pfenex's ability to manage operating expenses; Pfenex's ability to obtain additional funding to support its business activities and establish and maintain strategic business alliances and new business initiatives; Pfenex's dependence on third parties for development, manufacture, marketing, sales and distribution of products; unexpected expenditures; and difficulties in obtaining and maintaining intellectual property protection for its product candidates. Information on these and additional risks, uncertainties, and other information affecting Pfenex's business and operating results is contained in Pfenex's Annual Report on Form 10-K for the year ended December 31, 2015 and in Pfenex's subsequent reports on Form 10-Q and Form 8-K, filed with the Securities and Exchange Commission. Additional information will also be set forth in Pfenex's Quarterly Report on Form 10-Q for the period ended June 30, 2016 to be filed with the Securities and Exchange Commission. The forward-looking statements in this press release are based on information available to Pfenex as of the date hereof, and Pfenex disclaims any obligation to update any forward-looking statements, except as required by law.
Pfenex investors and others should note that we announce material information to the public about the Company through a variety of means, including our website (http://www.pfenex.com/), our investor relations website (http://pfenex.investorroom.com/), press releases, SEC filings, public conference calls, corporate Twitter account (https://twitter.com/pfenex), Facebook page (https://www.facebook.com/Pfenex-Inc-105908276167776/timeline/), and LinkedIn page (https://www.linkedin.com/company/pfenex-inc) in order to achieve broad, non-exclusionary distribution of information to the public and to comply with our disclosure obligations under Regulation FD. We encourage our investors and others to monitor and review the information we make public in these locations as such information could be deemed to be material information. Please note that this list may be updated from time to time.
About Pfenex Inc.
Pfenex Inc. is a clinical-stage biotechnology company engaged in the development of biosimilar therapeutics and high-value and difficult to manufacture proteins. The company's lead product candidate is PF582, a biosimilar candidate to Lucentis (ranibizumab), for the potential treatment of patients with retinal diseases. Pfenex has leveraged its Pfnex Expression Technology® platform to build a pipeline of product candidates and preclinical products under development including other biosimilars, as well as vaccines, therapeutic equivalents to reference listed drug products, and next generation biologics.
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201500011C.

Logo - http://photos.prnewswire.com/prnh/20140715/127348
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/pfenex-announces-positive-anthrax-vaccine-study-results-300310158.html
SOURCE Pfenex Inc.





