Magnetic muscle stimulation technology to strengthen, tone and firm muscles
MAGNETIC MUSCLE STIMULATION TECHNOLOGY TO STRENGTHEN, TONE AND FIRM MUSCLES
DUBLIN, June 24, 2019 /PRNewswire/ -- Allergan plc (NYSE: AGN) today announced CoolTone device received FDA clearance for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone is also indicated for strengthening, toning and firming of buttocks and thighs.

"As the global leader in medical aesthetics and body contouring, Allergan invests in the ongoing innovation and advancement of safe and effective non-surgical aesthetic solutions," said Brad Hauser, Vice President, R&D and General Manager, Body Contouring, Allergan. "We do this by developing differentiated technology, such as the CoolTone device, that helps meet the needs of our customers."
Using magnetic muscle stimulation (MMS), CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in improved muscle conditioning. Whether targeting abdomen, buttocks or thighs, CoolTone strengthens, tones and firms the muscles in the treated area, resulting in a more defined and toned appearance. CoolTone has 50 percent more magnetic intensity than the leading competitor (1.35 T versus 0.9 T) at the point of contact.* The clinical significance of this data has not been established.
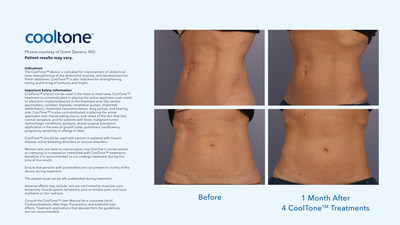
"By partnering with Allergan, I can offer my patients additional options in body contouring solutions," said Dr. Grant Stevens, Founder and Medical Director of Marina Plastic Surgery, Co-Founder and Chief Medical Officer of Orange Twist, and Chairman of the USC-Marina Aesthetic Surgery Fellowship. "CoolSculpting®, for non-surgical fat reduction, is one of the most requested treatments in my practice, and now I can introduce my patients to CoolTone for muscle toning, strengthening and firming of the abdomen, buttocks and thighs."
Allergan is now taking orders for the CoolTone device, and first units will ship early in the fourth quarter of this year.
*Based on performance testing measuring magnetic field expressed in tesla (T) over the applicator surface.
About the CoolTone Device
CoolTone uses magnetic muscle stimulation, or MMS technology, to penetrate into the muscle layers and induce involuntary muscle contractions. The body's response to these contractions is to strengthen its muscle fibers, resulting in improved muscle conditioning. CoolTone is FDA-cleared to strengthen, tone and firm the muscles of the abdomen, buttocks and thighs and is the latest addition to the Allergan body contouring portfolio of products. For more information about CoolTone, visit www.CoolTonebyCoolSculpting.com.
Indications for CoolTone
The CoolTone device is indicated for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone is also indicated for strengthening, toning and firming of buttocks and thighs.
Important Safety Information for CoolTone
CoolTone should not be used in the head or heart area. CoolTone treatment is contraindicated in placing the active applicator over metal or electronic implants/devices in the treatment area like cardiac pacemakers, cochlear implants, intrathecal pumps, implanted defibrillators, implanted neurostimulators, drug pumps, and hearing aids. CoolTone is also contraindicated in placing the active applicator over menstruating uterus, over areas of the skin that lack normal sensation, and for patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure application in the area of growth plate, pulmonary insufficiency, pregnancy, sensitivity or allergy to latex.
CoolTone should be used with caution in patients with Grave's disease, active bleeding disorders or seizure disorders.
Women who are close to menstruation may find that it comes sooner or cramping is increased or intensified with CoolTone treatments, therefore it is recommended to not undergo treatment during this time of the month.
Ensure that persons with pacemakers are not present in vicinity of the device during treatment.
The patient must not be left unattended during treatment.
Adverse effects may include, but are not limited to muscular pain, temporary muscle spasm, temporary joint or tendon pain, and local erythema or skin redness.
Consult the CoolTone User Manual for a complete list of Contraindications, Warnings, Precautions, and potential side effects. Treatment applications that deviate from the guidelines are not recommended.
About the CoolSculpting Treatment
CoolSculpting is a non-surgical, clinically proven treatment that selectively reduces unwanted fat using a patented cooling technology. Cleared by the FDA, CoolSculpting works by gently cooling targeted fat cells in the body to induce a natural, controlled elimination of fat cells without affecting surrounding tissue, and the treated fat cells are gone for good. In 2019, CoolSculpting was recognized for the sixth consecutive year by NewBeauty as a Choice Award winner. Millions of CoolSculpting treatments have been performed in more than 80 countries. CoolSculpting is available through a network of CoolSculpting Centers worldwide. Dermatologists, plastic surgeons and aesthetic specialists that offer CoolSculpting can be found at www.coolsculpting.com.
Indications for CoolSculpting
The CoolSculpting procedure is FDA-cleared for the treatment of visible fat bulges in the thigh, abdomen and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm in patients with a Body Mass Index (BMI) of ≤30 and in submental and submandibular areas in patients with a BMI of ≤46.2. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments.
Important Safety Information for CoolSculpting
CoolSculpting is contraindicated in patients with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Ask your patient about any medical conditions including recent surgery, pre-existing hernia, and any known sensitives or allergies.
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. Paradoxical hyperplasia (visibly enlarged tissue volume in the treated area) may develop 2-5 months after treatment and requires surgical intervention for correction.
As with any medical procedure, a consultation should be done by a licensed healthcare professional to determine if the patient is a candidate for treatment. Consult the CoolSculpting System User Manual for a complete list of Contraindications, Warnings, Precautions, and potential side effects. Treatment applicators that deviate from the guidelines are not recommended.
About Allergan plc
Allergan plc (NYSE: AGN), headquartered in Dublin, Ireland, is a global pharmaceutical leader focused on developing, manufacturing and commercializing branded pharmaceutical, device, biologic, surgical and regenerative medicine products for patients around the world. Allergan markets a portfolio of leading brands and best-in-class products primarily focused on four key therapeutic areas including medical aesthetics, eye care, central nervous system and gastroenterology. As part of its approach to delivering innovation for better patient care, Allergan has built one of the broadest pharmaceutical and device research and development pipelines in the industry.
With colleagues and commercial operations located in approximately 100 countries, Allergan is committed to working with physicians, healthcare providers and patients to deliver innovative and meaningful treatments that help people around the world live longer, healthier lives every day.
For more information, visit Allergan's website at www.Allergan.com.
Forward-Looking Statement
Statements contained in this press release that refer to future events or other non-historical facts are forward-looking statements that reflect Allergan's current perspective on existing trends and information as of the date of this release. Actual results may differ materially from Allergan's current expectations depending upon a number of factors affecting Allergan's business. These factors include, among others, the difficulty of predicting the timing or outcome of FDA approvals or actions, if any; the impact of competitive products and pricing; market acceptance of and continued demand for Allergan's products; the impact of uncertainty around timing of generic entry related to key products, including RESTASIS®, on our financial results; risks associated with divestitures, acquisitions, mergers and joint ventures; risks related to impairments; uncertainty associated with financial projections, projected cost reductions, projected debt reduction, projected synergies, restructurings, increased costs, and adverse tax consequences; difficulties or delays in manufacturing; and other risks and uncertainties detailed in Allergan's periodic public filings with the Securities and Exchange Commission, including but not limited to Allergan's Annual Report on Form 10-K for the year ended December 31, 2018 and Allergan's Quarterly Report on Form 10-Q for the period ended March 31, 2019. Except as expressly required by law, Allergan disclaims any intent or obligation to update these forward-looking statements.
|
CONTACTS: |
Allergan: |
|
Investors: |
|
|
Manisha Narasimhan, PhD |
|
|
(862) 261-7162 |
|
|
Christine Chiou |
|
|
(862) 261-7396 |
|
|
Media: |
|
|
Marlo Rodman |
|
|
(925) 918-1190 |
|
|
Amy Rose |
|
|
(862) 289-3072 |


![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/allergan-receives-fda-clearance-for-cooltone-device-300873208.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/allergan-receives-fda-clearance-for-cooltone-device-300873208.html
SOURCE Allergan plc
Company Codes: NYSE:AGN




