Updated biologics-related tools and statements further validate the importance of evidence-based information to help guide the musculoskeletal health community [14-October-2020] ROSEMONT, Ill. , Oct. 14, 2020 /PRNewswire/ -- The American Academy of Orthopaedic Surgeons (AAOS) continues to demonstrate its commitment to advancing the quality of musculoskeletal care in
|
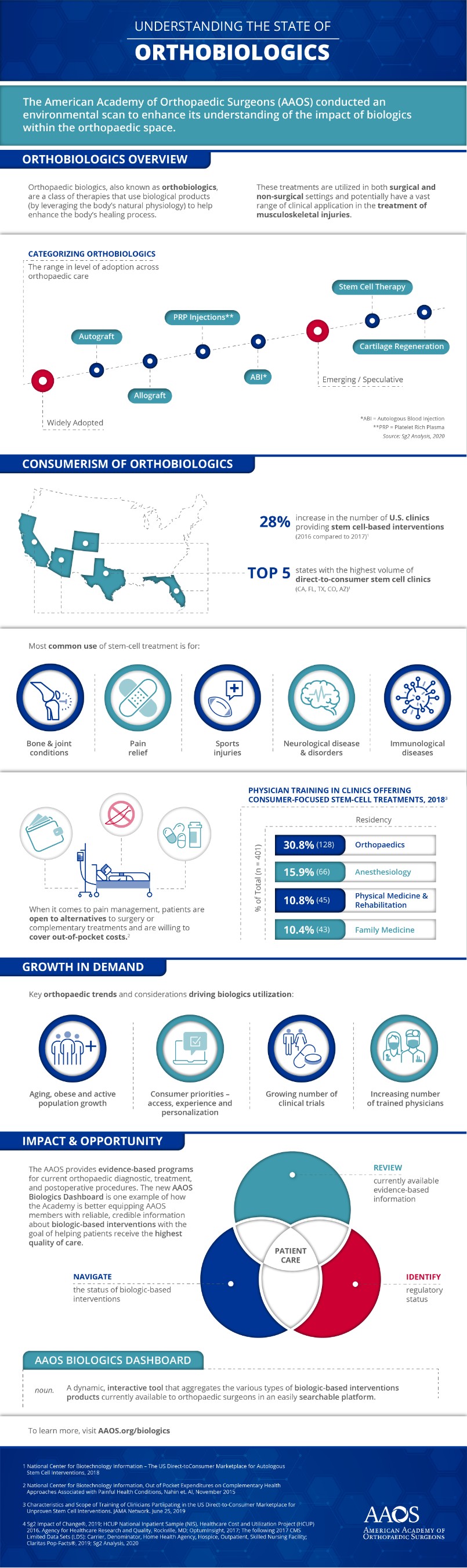
ROSEMONT, Ill., Oct. 14, 2020 /PRNewswire/ -- The American Academy of Orthopaedic Surgeons (AAOS) continues to demonstrate its commitment to advancing the quality of musculoskeletal care in a fully transparent and scientific way. Debuting today as a new member benefit, the AAOS Biologics Dashboard is a dynamic online tool designed to help orthopaedic surgeons navigate the approval status of biologic-based interventions. The development of the AAOS Biologics Dashboard is just one of several efforts within the Academy's Biologics Initiative that offers evidence-based guidance to the musculoskeletal health community. An additional effort is the revision of two biologics-related position statements, recently approved by the AAOS Board of Directors. Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8776851-aaos-biologics-dashboard/ "Orthobiologics is an evolving frontier. As new therapies, such as regenerative medicine therapies and stem-cell injections, become increasingly popular due to their potential to regenerate tissue and enhance bone healing and reduce pain, the AAOS continues to recognize a need for reliable and credible sources of evidence-based information," said David S. Jevsevar, MD, MBA, FAAOS, Chair of the AAOS Committee on Devices, Biologics & Technology. "These new tools further validate AAOS' leadership in separating science from hope with the goal of helping patients receive the highest quality of evidence-based care." Understanding the Safety and Efficacy of Orthobiologic Interventions "Until now, there was not a tool or platform that could help our members clearly navigate emergent and established biologic-based products, the evidence which has accompanied their coming to market, and the various FDA approval pathways that exist for these types of products," added Dr. Jevsevar. "We are excited to now be able to synthesize multiple regulatory guidance documents and principles with only a few clicks." The AAOS Biologics Dashboard allows the user to enter four characteristics of the product, including the tissue type the product is derived from, whether it is autograft (a patient's self), allograft (from another person), or xenograft (from an animal), how the product is processed, and what use the user is considering for the product. Once inputted, the dashboard will reveal a red, yellow or green color indicator to help visually illustrate the regulatory status of the orthobiologics product shown. While not intended to be a definitive end point or imply endorsement, efficacy, or appropriateness for use by the AAOS, the color indicator offers guidance, rationale, and reference in one convenient tool. As available evidence and federal guidance evolve, and new products come on the market, the AAOS Biologics Dashboard will also continue to be updated and improved to best serve AAOS members and their patients. Updated Position Statements
"As the world's largest medical association of musculoskeletal specialists, we have a tremendous opportunity and responsibility to educate our patients about available evidence for orthobiologics treatments and to vastly improve patient care within this space," said Martha Murray, MD, FAAOS, Professor of Orthopaedic Surgery at Boston Children's Hospital and Harvard Medical School and Committee on Devices, Biologics & Technology member. "By updating these AAOS position statements with standardized, modern language, we are able to better hold our specialty accountable with reference to current FDA regulatory pathways." For more information about the AAOS' efforts in orthobiologics, visit www.aaos.org/biologics. To speak with a member of the DBT Committee about how the AAOS is leading the way in evidence-based medicine as it pertains to the field of orthobiologics and the future of patient care or to request media access to the AAOS Biologics Dashboard, email media@aaos.org. About the AAOS More information about the AAOS

SOURCE American Academy of Orthopaedic Surgeons |




