Antengene Corporation Limited (“Antengene”, SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing, and commercializing global first-in-class and/or best-in-class therapeutics in hematology and oncology, presented preclinical data at the American Association for Cancer Research (AACR) Annual Meeting 2021,
|
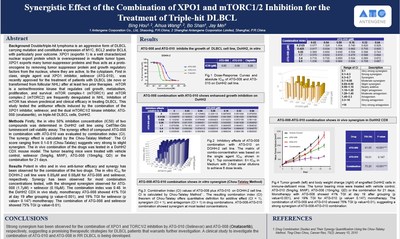
SHANGHAI, China and GAITHERSBURG, U.S., April 12, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing, and commercializing global first-in-class and/or best-in-class therapeutics in hematology and oncology, presented preclinical data at the American Association for Cancer Research (AACR) Annual Meeting 2021, which demonstrated the synergistic effect of the combination of ATG-010 (selinexor, XPO1 inhibitor) and ATG-008 (onatasertib, mTORC1/2 inhibitor) for the treatment of triple-hit diffuse large B-cell lymphoma (DLBCL). Results from this study demonstrated potent in vitro and in vivo anti-tumor efficacy and synergy with the combination of ATG-010 and ATG-008, including strong synergistic activities in the triple-hit DLBCL cell line. Meanwhile, in the DLBCL circulating tumor cell-derived explants (CDX) model, the combination of ATG-010 and ATG-008 also showed enhanced tumor growth inhibition and synergism. The single agent oral XPO1 inhibitor, ATG-010, is a first-in-class and only-in-class selective inhibitor of nuclear export (SINE) compound, approved by the US Food and Drug Administration (FDA) for the treatment of patients with DLBCL after at least two prior therapies. ATG-008 is a dual mTORC1/2 kinase inhibitor, which has shown preclinical and clinical activity in treating DLBCL. Antengene is currently developing a clinical study to investigate the combination of ATG-010 and ATG-008 in relapsed or refractory DLBCL (the MATCH trial). The preclinical data showed:
Dr. Jay Mei, Founder, Chairman and CEO of Antengene, commented: "ATG-010 has been approved for the treatment of relapsed or refractory DLBCL, whilst ATG-008 has shown early clinical activity in the same disease. Using in vitro and in vivo DLBCL models, we found that ATG-010 combined with ATG-008 at certain concentrations could achieve amplified anti-tumor activity in triple-hit DLBCL. The strong synergism of the ATG-010 plus ATG-008 combination suggests a promising therapeutic strategy for the treatment of patients with DLBCL, including triple-hit disease where significant unmet medical needs exist, that we are looking forward to exploring in the MATCH trial." About Antengene Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) is a leading clinical-stage R&D driven biopharmaceutical company focused on innovative medicines for oncology and other life threatening diseases. Antengene aims to provide the most advanced anti-cancer drugs to patients in the Asia Pacific Region and around the world. Since its establishment in 2017, Antengene has built a broad and expanding pipeline of clinical and pre-clinical stage assets through partnerships as well as in-house drug discovery, and obtained 13 investigational new drug (IND) approvals and submitted 5 new drug applications (NDA) in multiple markets in Asia Pacific. Antengene's vision is to "Treat Patients Beyond Borders". Antengene is focused on and committed to addressing significant unmet medical needs by discovering, developing and commercializing first-in-class/best-in-class therapeutics. For more information, please visit: www.antengene.com. Forward-looking statements The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
SOURCE Antengene Corporation Limited |
||
Company Codes: HongKong:6996 |