ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal technologies for treating patients with acute respiratory failure, announced the recent initiation of commercial development of its next generation artificial lung, which expands the Company’s focus on highly efficient gas exchange devices and also broadens its applicable market.
PITTSBURGH--(BUSINESS WIRE)-- ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced the recent initiation of commercial development of its next generation artificial lung, which expands the Company’s focus on highly efficient gas exchange devices and also broadens its applicable market.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200505005548/en/
(Photo: Business Wire)
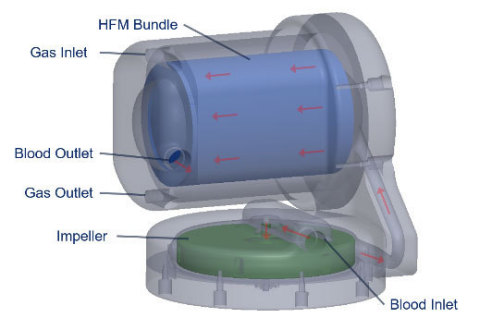
The Company’s current product, the Hemolung® Respiratory Assist System (RAS), is the only fully comprehensive extracorporeal carbon dioxide removal (ECCO2R) system specifically designed and manufactured for this therapy, as compared to complex competitive products that are modifications of existing technologies designed for other purposes. The Hemolung continues to be the most highly efficient and simple to use ECCO2R system on the market today.
The next generation Hemolung RAS is based upon intellectual property recently licensed to ALung from the University of Pittsburgh. Developed by Professor William Federspiel, PhD and colleagues at the Swanson School of Engineering and the McGowan Institute for Regenerative Medicine, this new technology platform significantly enhances gas exchange efficiency while reducing the deleterious hematologic effects from extracorporeal blood circulation. The licensed research was supported in part by the National Institutes of Health and the Coulter Translational Research Partners II Program at the University of Pittsburgh. Dr. Federspiel has an equity holding in the company and is compensated as an advisory board member.
“The next generation Hemolung RAS is a direct result of the continued collaboration between the University of Pittsburgh and ALung Technologies. This collaboration, spanning 20+ years, has resulted in a rich pipeline of innovation for ALung that will accelerate the development of highly efficient, simple to use artificial lung devices for the treatment of acute respiratory failure. The foundation of our next generation system is an integrated artificial lung cartridge/blood pump that will be unparalleled in the industry as the most efficient carbon dioxide removal and oxygen delivery system, which will address the needs of acute respiratory failure patients that require ECCO2R and/or ECMO (extracorporeal membrane oxygenation). All of this will again be consolidated in a comprehensive, easy to use system without all of the complexities represented in competitive systems,” stated Peter M. DeComo, Chairman and CEO of ALung Technologies.
Jeremy Kimmel, PhD, Vice President of New Technology at ALung Technologies stated, “Professor Federspiel and colleagues at the University of Pittsburgh have rapidly advanced this technology toward commercial readiness through state of the art computational, in vitro and in vivo testing, including successful 7-day and 30-day large animal studies. ALung has initiated commercial development of the next generation Hemolung RAS to provide clinicians with the flexibility to support patients across the full spectrum of acute and acute-on-chronic respiratory failure using a single integrated device. The system design will accommodate bedside therapy as well as portability and wearability, further enhancing device usability and expanding potential clinical indications.”
Key features and benefits of the next generation Hemolung RAS will include:
- Patent-pending technology that generates superior blood flow uniformity to maximize gas exchange efficiency.
- A custom designed centrifugal pump integrated with a low surface area (0.65 m2) gas exchange membrane without the need for additional components (e.g. heat exchanger, pressure ports) that will reduce operational complexity of the system.
- Low flow ECCO2R (250 – 700 mL/min) as well as full ECMO (2 – 4 L/min) using a single integrated pump and gas exchange membrane.
- The highest efficiency oxygenation of any ECMO device on the market providing full oxygen saturation at ≤4 L/min blood flow with membrane surface area of 0.65 m2.
COPD affects 30 million Americans1 and is the third leading cause of death in the United States behind cancer and heart disease.2 Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. ARDS is estimated to affect more than 10% of intensive care unit patients globally, has a mortality rate as high as 45% and requires invasive mechanical ventilation in the majority of cases.3,4 Combined, these disorders represent a significant need and a global market for innovative respiratory assist devices. The COVID-19 pandemic is a recent example of such a dramatic need.
Currently, the Hemolung RAS has European marketing clearance (CE Mark). In addition, it is the only system that has been studied for safety and efficacy in two large landmark pivotal trials; the FDA approved VENT-AVOID trial and the U.K. REST trial. The Hemolung RAS was recently granted Emergency Use Authorization (EUA) by FDA for the treatment of acute respiratory failure caused by COVID-19.
About ALung Technologies
ALung Technologies, Inc. is a privately held Pittsburgh-based developer and manufacturer of innovative lung assist devices. Founded in 1997 as a spin-out of the University of Pittsburgh, ALung has developed the Hemolung RAS as a dialysis-like alternative or supplement to mechanical ventilation. ALung is backed by Philips, UPMC Enterprises, Abiomed, The Accelerator Fund, Allos Ventures, Birchmere Ventures, Blue Tree Ventures, Eagle Ventures, Riverfront Ventures, West Capital Advisors, and other individual investors.
For more information about ALung and the Hemolung RAS, visit www.alung.com.
For more information on the VENT-AVOID trial, and a list of enrolling sites, please visit clinicaltrials.gov.
For more information about the REST Trial, please visit UK National Institute for Health Research (NIHR) – REST Trial Project Website.
For more information on the use of the Hemolung RAS for COVID-19 patients, please visit https://www.alung.com/covid-19/covid-19-us/
CAUTION: The Hemolung RAS is an Investigational Device and limited by United States law to investigational use.
This press release may contain forward-looking statements, which, if not based on historical facts, involve current assumptions and forecasts as well as risks and uncertainties. Our actual results may differ materially from the results or events stated in the forward-looking statements, including, but not limited to, certain events not within the Company’s control. Events that could cause results to differ include failure to meet ongoing developmental and manufacturing timelines, changing GMP requirements, the need for additional capital requirements, risks associated with regulatory approval processes, adverse changes to reimbursement for the Company’s products/services, and delays with respect to market acceptance of new products/services and technologies. Other risks may be detailed from time to time, but the Company does not attempt to revise or update its forward-looking statements even if future experience or changes make it evident that any projected events or results expressed or implied therein will not be realized.
References
1. https://www.copdfoundation.org/What-is-COPD/COPD-Facts/Statistics.aspx
2. http://www.lung.org/assets/documents/research/copd-trend-report.pdf
3. Bellani. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788-800.
4. Walkey A. Acute respiratory distress syndrome: epidemiology and management approaches. Clinical Epidemiology 2012:4 159–169.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200505005548/en/
ALung Technologies, Inc.
Peter M. DeComo
Chairman and CEO
+1-412-697-3370 ext. 207
pdecomo@alung.com
Source: ALung Technologies, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20200505005548/en