First and only company to receive FDA authorization to market a direct-to-consumer genetic test
MOUNTAIN VIEW, Calif., Oct. 21, 2015 /PRNewswire/ -- 23andMe, Inc., the leading personal genetics company, today announced the launch of their new Personal Genome Service (PGS). Following two years of work with the FDA, extensive user comprehension testing and a complete redesign, 23andMe is launching an entirely new experience that includes carrier status, wellness, trait and ancestry reports.
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7665351-23andme-new-experience/
"We've worked with the FDA for nearly two years to establish a regulatory path for direct-to-consumer genetic testing. We are a better company with a better product as a result of our work with the FDA," said 23andMe co-founder and CEO Anne Wojcicki. "This is an incredibly dynamic time in genetics and we're excited to be at the leading edge of bringing genetics directly to individuals as they begin to learn about their 23 pairs of chromosomes."
The service now delivers more tools, insights and better functionality. For $199, customers receive a detailed, but easy to understand genetic information service, validated by user testing:
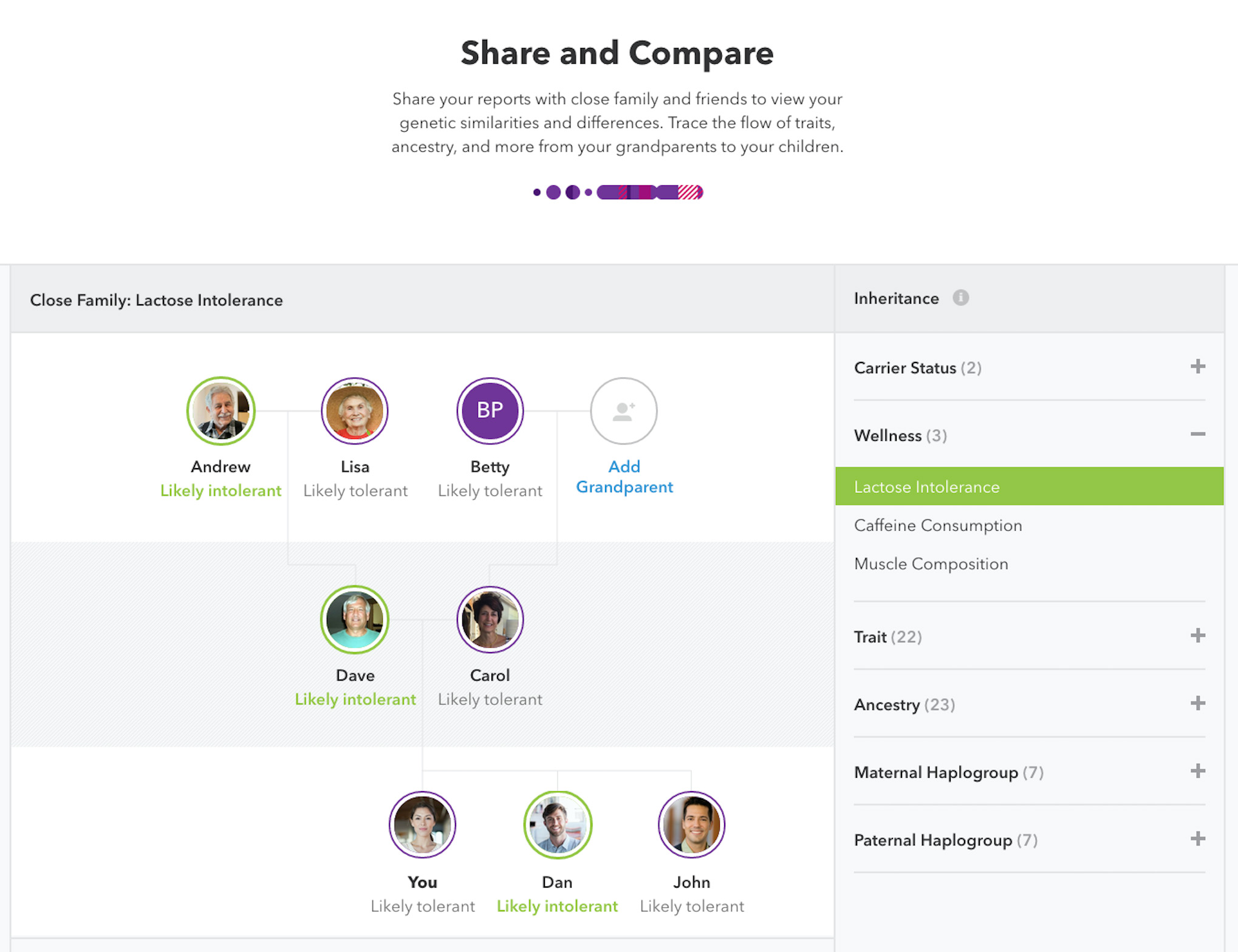
- More than 60 health, ancestry, wellness, and personal trait reports
- Reports on genetic research and new genetic discoveries
- Personalized insights based on analysis of 650,000 genetic variations
- Only service available direct-to-consumer with reports that meet FDA standards
- Tools and features unique to 23andMe, such as segment level data for advanced genetic genealogy research and other patented features
- The chance to find and connect with DNA relatives in a database of more than 1 million customers
- New or updated reports, at no additional cost as part of the PGS, as genetic discoveries are made and as we are able to clear new reports for our genotyping service through the FDA
Existing customers will be transitioned to the new experience as quickly as possible. The transition of some customers will be dependent on validation and local requirements.
"23andMe is more than a test or series of reports, it's a platform that offers individuals a new way to look at themselves through their genetics. We have worked closely with scientists, leaders in the medical community and regulators to develop a product that provides personalized, highly relevant information to individuals," said Andy Page, President of 23andMe. "Our new product represents an extraordinary effort from our regulatory and quality teams, and an incredible group of 23andMe scientists, product designers, researchers, and engineers, all focused on an exceptional customer experience."
In addition to the new reports, features and tools, 23andMe's Personal Genome Service provides customers with the opportunity to participate in research that holds the promise of accelerating the pace of discovery and improving the understanding of how genetics influences our lives. Those who consent to participate can easily contribute from anywhere by answering survey questions online and, in turn, receive insights along the way to help learn more about their genetics, see early findings from 23andMe research and learn how they compare to others. A new 23andMe Research mobile app will also be available on the Apple App Store.
The new 23andMe Personal Genome Service can be purchased online atwww.23andMe.com. Current customers do not have to pay additional fees for the new reports.
Product and platform photos and b-roll can be accessed here.
Product videos available here.
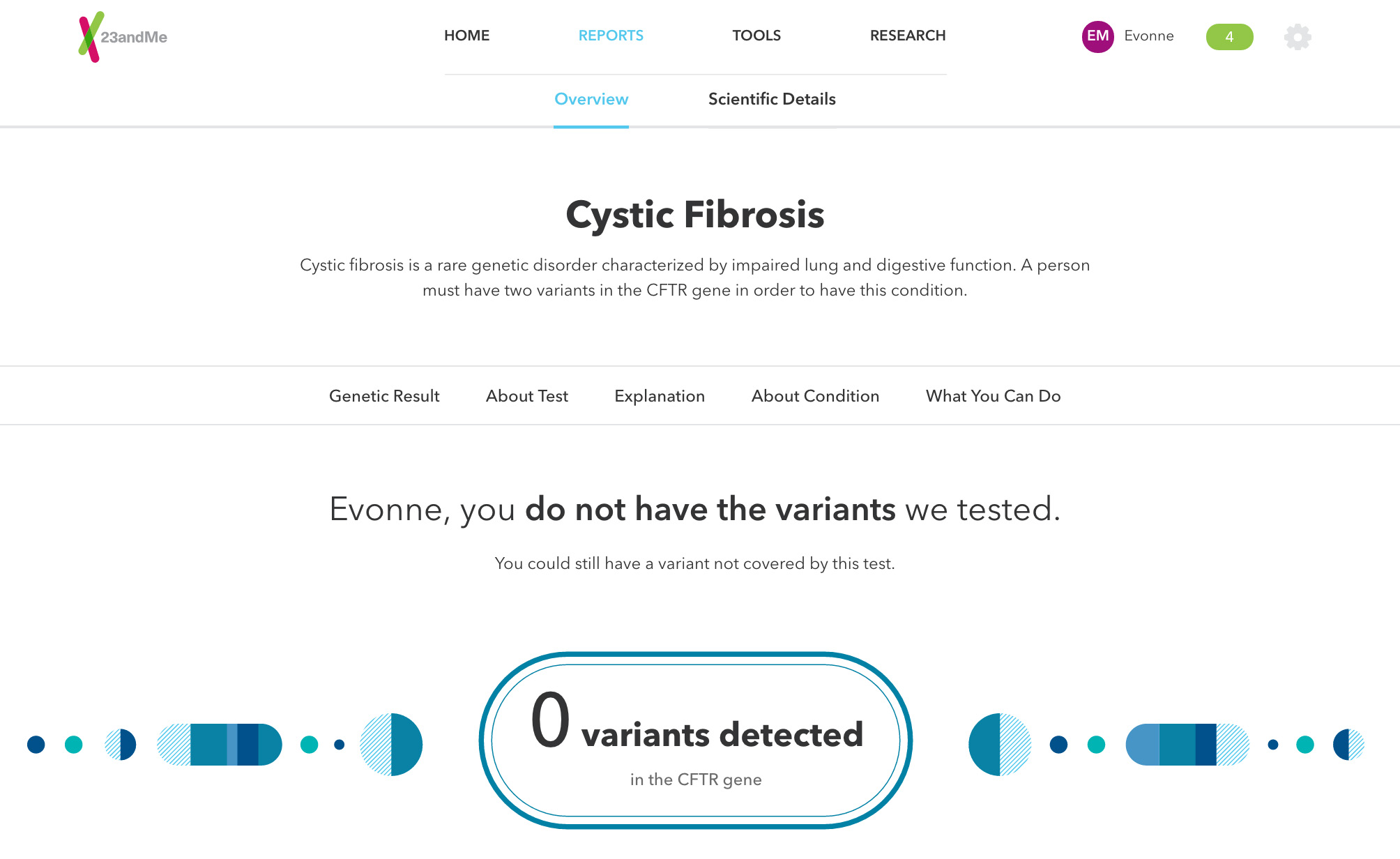
About Carrier Status Tests
[Our tests] can be used to determine carrier status in adults from saliva collected using an FDA-cleared collection device (Oragene DX model OGD.500.001), but cannot determine if you have two copies of the genetic variant. Each test is most relevant for people of certain ethnicities. The tests are not intended to diagnose a disease, or tell you anything about your risk for developing a disease in the future. On their own, carrier status tests are not intended to tell you anything about the health of your fetus, or your newborn child's risk of developing a particular disease later in life.
About 23andMe
23andMe, Inc. is the leading personal genetics company. Founded in 2006, the mission of the company is to help people access, understand and benefit from the human genome. 23andMe has over one million customers worldwide with over 80 percent consented to participate in research. 23andMe, Inc. is located in Mountain View, CA. More information is available at www.23andMe.com.
Media@23andMe.com
1-800-390-3398
Angela Calman-Wonson
Angela@23andMe.com
(212) 729-8088
Lindsay Evans
Lindsay.Evans@Edelman.com
(646) 234-0011

To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/23andme-launches-new-customer-experience---reports-include-carrier-status-that-meet-fda-standards-wellness-traits-and-ancestry-300163671.html
SOURCE 23andMe, Inc.
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




