- UltraShape Power provides a 20% increase in Acoustic Energy; Demonstrates 32% reduction in abdominal fat layer thickness
WAYLAND, Mass., July 18, 2016 /PRNewswire/ -- Syneron Medical Ltd. (NASDAQ: ELOS), a leading global aesthetic device company, announces the U.S. Food and Drug Administration (FDA) clearance of its non-invasive fat destruction device, UltraShape Power. The FDA clearance is for non-invasive reduction of abdominal circumference via fat cell destruction.
UltraShape Power uses focused, pulsed mechanical ultrasound energy to target and destroy fat, offering measurable fat reduction to the abdominal area. UltraShape Power's advanced USculpt transducer delivers 20% more energy than its predecessor. A recent clinical study with UltraShape Power's USculpt transducer documents a 32% reduction in subcutaneous fat thickness, positioning UltraShape Power as a powerful solution for non-invasive fat reduction.
"UltraShape Power represents the latest advancement in non-thermal focused ultrasound for fat destruction. The device's unique deployment of higher acoustic energy via its new transducer allows for the precise targeting of small and large pockets of fat with minimal increase in tissue temperature, resulting in a comfortable treatment experience. The outcomes observed using UltraShape Power have been positive and our patient experience has been very satisfactory," says Suzanne Kilmer, MD, FAAD, Founding Director of the Laser & Skin Surgery Center of Northern California.
UltraShape Power's ultrasound energy is applied to the skin in a proprietary pulse structure to ensure effective fat destruction with no damage to surrounding tissue including blood vessels, nerves and muscles, resulting in a safe and comfortable treatment experience. UltraShape Power's lighter transducer for high maneuverability enables the customized treatment of large and small fat pockets. The device also incorporates a sophisticated treatment and patient management software package combined with flexible communication options.
"The new UltraShape Power system allows for fully customizable treatments that meet the unique needs of each patient," says Alix Charles, M.D., FAAD, clinical trial investigator, from Dupage Medical Group, Hinsdale, IL. "The clinical studies show a pain score of less than one out of a ten point scale, and my patients have experienced similar comfort levels."
"We are pleased to announce that we have received FDA clearance for UltraShape Power in the US. UltraShape Power's strong market acceptance since its launch in the second quarter 2016 outside of the U.S. has been driven by its powerful non-invasive fat reduction capability, patient comfort and an emphasis on ease of use. We believe that this new generation of UltraShape will have a significant impact on our ability to continue building our global leadership position in the fast growing, non-invasive fat destruction market," says Amit Meridor, CEO of Syneron Candela.
Discussing the third quarter 2016 market launch of the UltraShape Power in the United States, Jeff Nardoci, President, North American Body Group says, "We look forward to launching our new UltraShape Power campaign following the ramp up of our installations. Initial testing outcomes indicate that the campaign resonates well with consumers and carries through with our professional audience as well. The UltraShape Power's increased energy provides greater efficacy and high comfort, while the device's advanced treatment modes allow us to treat a full abdomen in close to 30 minutes. WiFi connectivity allows for remote diagnostics and scheduling support. We believe the combination of these features provides the professional market with a very strong value proposition in the arena of non-invasive fat destruction," he concludes.
About Syneron Candela:
Syneron Candela is a leading global aesthetic device company with a comprehensive product portfolio and a global distribution footprint. The Company's technology enables physicians to provide advanced solutions for a broad range of medical-aesthetic applications including body contouring, hair removal, wrinkle reduction, tattoo removal, improving the skin's appearance through the treatment of superficial benign vascular and pigmented lesions, and the treatment of acne, leg veins and cellulite. The Company has a wide portfolio of trusted, leading products including UltraShape, VelaShape, CO2RE, CO2RE Intima, GentleLase, VBeam Perfecta, PicoWay, Profound and elos Plus.
Founded in 2000, the corporate, R&D, and manufacturing headquarters for Syneron Candela are located in Israel. Syneron Candela also has R&D and manufacturing operations in the U.S. The company markets, services and supports its products in 86 countries. It has offices in North America, France, Germany, Italy, Portugal, Spain, UK, Australia, China, Japan, and Hong Kong and distributors worldwide.
SAFE HARBOR FOR FORWARD-LOOKING STATEMENTS
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. Such forward-looking statements include the expectations, plans and prospects for the Company, including potential clinical successes, market acceptance of new products, and projected revenues, margins, earnings and market shares. The statements made by the Company are based upon management's current expectations and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include the risk factors and other cautionary statements described in the Company's filings with the SEC, including those described in the Company's most recent Annual Report on Form 20-F, and in the filings that Syneron Medical makes with the SEC, and other factors beyond the Company's control. If one or more of these factors materialize, or if any underlying assumptions prove incorrect, Syneron Medical Ltd.'s actual results, performance or achievements may vary materially from those expressed or implied by these forward-looking statements. These forward-looking statements should not be relied upon as representing Syneron Medical Ltd.'s views as of any date after the date of this document. The Company does not intend to update these statements and undertakes no duty to any person to provide any such update under any circumstance.
For additional information, please visit http://www.syneron-candela.com.
Media contacts:
Kim Angelastro, Cohn & Wolfe
E: Kim.Angelastro@cohnwolfe.com
M: 912.441.8263
P: 212.798.9740
Investor Relations contact:
Zack Kubow, The Ruth Group
E: zkubow@theruthgroup.com
P: 646-536-7020
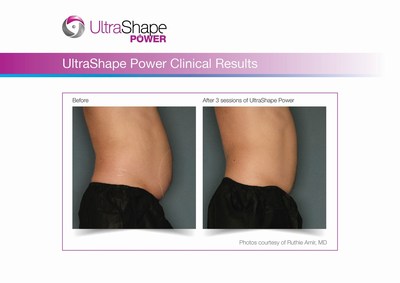
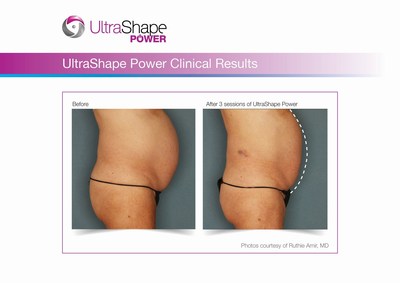

Photo - http://photos.prnewswire.com/prnh/20160718/390403
Photo - http://photos.prnewswire.com/prnh/20160718/390402
Photo - http://photos.prnewswire.com/prnh/20160718/390404
Logo - http://photos.prnewswire.com/prnh/20120528/535447LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/syneron-candela-announces-fda-clearance-of-high-energy-ultrashape-power-for-fat-destruction-300299830.html
SOURCE Syneron Medical Ltd.





