Clovis Oncology, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved Rubraca® (rucaparib) tablets for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy.
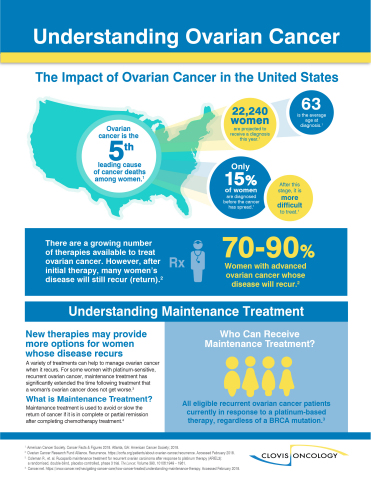
Infographic: Understanding Ovarian Cancer (Graphic: Business Wire)
In addition to granting Rubraca approval in this second indication, the FDA converted the approval of the initial treatment indication from accelerated to regular approval.
“Rubraca provided statistically-significant improvement in PFS versus placebo to all patients, regardless of BRCA mutation status,” said Robert L. Coleman, MD, Professor & Executive Director, Cancer Network Research, Ann Rife Cox Chair in Gynecology, Department of Gynecologic Oncology and Reproductive Medicine at University of Texas MD Anderson Cancer Center in Houston and one of the Principal Investigators in the ARIEL3 clinical trial program. “Both the efficacy and safety results from the ARIEL3 study reinforce the important role of Rubraca in the treatment of recurrent ovarian cancer and expands the treatment options for patients and physicians battling this disease.”
“This FDA approval provides a meaningful advancement for the treatment of women with recurrent ovarian cancer, offering them the potential to reduce their risk of disease progression following platinum-based chemotherapy,” said Patrick J. Mahaffy, CEO and President of Clovis Oncology. “We are grateful that the FDA expedited review of this maintenance treatment indication, so that physicians can begin offering it to appropriate patients beginning today.”
On February 28, 2018, Rubraca was added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Ovarian Cancer, as maintenance therapy for patients with platinum-sensitive epithelial ovarian, fallopian tube and primary peritoneal cancer who are in partial or complete response after completion of two or more lines of platinum-based therapy. The NCCN designated Rubraca as a category 2A treatment.
NCCN is a not-for-profit alliance that includes 27 of the world’s leading cancer institutions. The NCCN Guidelines document evidence-based, consensus-driven management to ensure that all patients receive preventive, diagnostic, treatment, and supportive services that are most likely to lead to optimal outcomes.[1]
In December 2017, FDA accepted the Rubraca supplemental New Drug Application (sNDA) application and granted priority review status. Priority review designation is granted to proposed medicines that FDA has determined have the potential, if approved, to offer a significant improvement in the safety or effectiveness for the treatment, prevention or diagnosis of a serious condition when compared to standard applications. The Rubraca maintenance treatment approval is based on positive results from the ARIEL3 study, which evaluated Rubraca in the ovarian cancer maintenance-treatment setting among three populations: 1) BRCA mutant (BRCAmut+) 2) HRD positive inclusive of BRCAmut+ and, 3) all patients treated in ARIEL3. The study enrolled a total of 564 patients.
ARIEL3 successfully achieved both its primary and key secondary endpoints, extending investigator assessed progression-free survival (PFS) versus placebo in all patients treated, regardless of BRCA status.
| Parameter | Investigator Assessment | |||||||||||||||
| Rubraca | Placebo | |||||||||||||||
| All Patientsa | ||||||||||||||||
| Patients, N | 375 | 189 | ||||||||||||||
| PFS events, n (%) | 234 (62%) | 167 (88%) | ||||||||||||||
| PFS, median in months | 10.8 | 5.4 | ||||||||||||||
| HR (95% CI) | 0.36 (0.30, 0.45) | |||||||||||||||
| p-value | <0.0001 | |||||||||||||||
| tBRCA Groupb | ||||||||||||||||
| Patients, N | 130 | 66 | ||||||||||||||
| PFS events, n (%) | 67 (52%) | 56 (85%) | ||||||||||||||
| PFS, median in months | 16.6 | 5.4 | ||||||||||||||
| HR (95% CI) | 0.23 (0.16, 0.34) | |||||||||||||||
| p-value | <0.0001 | |||||||||||||||
| a. | All randomized patients | |||||||||||
| b. |
tBRCA (tumor BRCA) includes all patients with a deleterious germline or somatic BRCA mutation, as determined by the CTA. |
|||||||||||
Clovis announced topline results from the ARIEL3 clinical trial in June 2017. Additional data from the trial were presented at the 2017 European Society for Medical Oncology (ESMO) Annual Conference in Madrid, Spain, and subsequently published in The Lancet.
“The FDA approval of Rubraca in the maintenance treatment setting is an important milestone for physicians and their patients with recurrent ovarian cancer because it offers them greater flexibility to use this novel PARP inhibitor, which has demonstrated significant clinical efficacy and has been well received in practice,” said Professor Jonathan Ledermann, MD, Professor of Medical Oncology, Clinical Director, UCL Cancer Institute, and European and the rest of world Principal Investigator for the ARIEL3 study. “This will enable physicians to offer Rubraca to more women with platinum-sensitive, recurrent ovarian cancer.”
"Tens of thousands of women will battle ovarian cancer every year,” said David Barley, Chief Executive Officer, National Ovarian Cancer Coalition. “We need therapies that provide clinically meaningful improvements in reducing the risk of disease progression, among women with recurrent disease."
The safety evaluation of Rubraca 600 mg twice daily as monotherapy for maintenance treatment is based on data from 561 patients with recurrent ovarian cancer treated in the ARIEL3 trial. The safety and tolerability of Rubraca observed in this study was consistent with the previous Rubraca studies. The most common adverse reactions (greater than or equal to 20% of patients; CTCAE Grade 1-4) were nausea, fatigue/asthenia, abdominal pain/distention, rash, dysgeusia, anemia, AST/ALT elevation, constipation, vomiting, diarrhea, thrombocytopenia, nasopharyngitis/upper respiratory tract infection, stomatitis, decreased appetite and neutropenia. The most common laboratory abnormalities (greater than or equal to 25% of patients; CTCAE Grade 1-4) were increase in creatinine, decrease in hemoglobin, increase in cholesterol, increase in alanine aminotransferase (ALT), increase in increase in aspartate aminotransferase (AST), decrease in platelets, decrease in leukocytes, decrease in neutrophils, increase in alkaline phosphatase and decrease in lymphocytes. The majority of adverse reactions and laboratory abnormalities were Grade 1-2.
About Rubraca Connections
Rubraca is available in the United States through specialty pharmacies and distributors. Clovis is committed to ensuring Rubraca access for patients and offers eligible patients financial and reimbursement support through Rubraca Connections. More information about Rubraca Connections is available at RubracaConnections.com or by calling 1-844-779-7707 between 8 a.m. and 8 p.m. Eastern Time, Monday through Friday.
About Rubraca® (rucaparib)
Rubraca is an oral, small molecule inhibitor of PARP1, PARP2 and PARP3 being developed in ovarian cancer as well as several additional solid tumor indications. Studies open for enrollment or under consideration include ovarian, prostate, breast, gastroesophageal, pancreatic, lung and bladder cancers. Clovis holds worldwide rights for Rubraca.
In the United States, Rubraca is approved for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy. Rubraca is also approved in the United States for the treatment of adult patients with deleterious BRCA mutation (germline and/or somatic) associated epithelial ovarian, fallopian tube, or primary peritoneal cancer who have been treated with two or more chemotherapies, and selected for therapy based on an FDA-approved companion diagnostic for Rubraca.
Rubraca is an unlicensed medical product outside of the U.S.
Select Important Safety Information
Myelodysplastic Syndrome (MDS)/Acute Myeloid Leukemia (AML) occur uncommonly in patients treated with Rubraca, and are potentially fatal adverse reactions. In approximately 1100 treated patients, MDS/AML occurred in 12 patients (1.1%), including those in long term follow-up. Of these, 5 occurred during treatment or during the 28 day safety follow-up (0.5%). The duration of Rubraca treatment prior to the diagnosis of MDS/AML ranged from 1 month to approximately 28 months. The cases were typical of secondary MDS/cancer therapy-related AML; in all cases, patients had received previous platinum-containing regimens and/or other DNA damaging agents.
Do not start Rubraca until patients have recovered from hematological toxicity caused by previous chemotherapy (≤ Grade 1).
Monitor complete blood counts for cytopenia at baseline and monthly thereafter for clinically significant changes during treatment. For prolonged hematological toxicities (> 4 weeks), interrupt Rubraca or reduce dose (see Dosage and Administration (2.2) in full Prescribing Information) and monitor blood counts weekly until recovery. If the levels have not recovered to Grade 1 or less after 4 weeks or if MDS/AML is suspected, refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics. If MDS/AML is confirmed, discontinue Rubraca.
Based on its mechanism of action and findings from animal studies, Rubraca can cause fetal harm when administered to a pregnant woman. Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of Rubraca.
Most common adverse reactions in ARIEL3 (≥ 20%; Grade 1-4) were nausea (76%), fatigue/asthenia (73%), abdominal pain/distention (46%), rash (43%), dysgeusia (40%), anemia (39%), AST/ALT elevation (38%), constipation (37%), vomiting (37%), diarrhea (32%), thrombocytopenia (29%), nasopharyngitis/upper respiratory tract infection (29%), stomatitis (28%), decreased appetite (23%), and neutropenia (20%).
Most common laboratory abnormalities in ARIEL3 (≥ 25%; Grade 1-4) were increase in creatinine (98%), decrease in hemoglobin (88%), increase in cholesterol (84%), increase in alanine aminotransferase (ALT) (73%), increase in aspartate aminotransferase (AST) (61%), decrease in platelets (44%), decrease in leukocytes (44%), decrease in neutrophils (38%), increase in alkaline phosphatase (37%), and decrease in lymphocytes (29%).
Most common adverse reactions in Study 10 and ARIEL2 (≥ 20%; Grade 1-4) were nausea (77%), asthenia/fatigue (77%), vomiting (46%), anemia (44%), constipation (40%), dysgeusia (39%), decreased appetite (39%), diarrhea (34%), abdominal pain (32%), dyspnea (21%), and thrombocytopenia (21%).
Most common laboratory abnormalities in Study 10 and ARIEL2 (≥ 35%; Grade 1-4) were increase in creatinine (92%), increase in alanine aminotransferase (ALT) (74%), increase in aspartate aminotransferase (AST) (73%), decrease in hemoglobin (67%), decrease in lymphocytes (45%), increase in cholesterol (40%), decrease in platelets (39%), and decrease in absolute neutrophil count (35%).
Co-administration of rucaparib can increase the systemic exposure of CYP1A2, CYP3A, CYP2C9, or CYP2C19 substrates, which may increase the risk of toxicities of these drugs. Adjust dosage of CYP1A2, CYP3A, CYP2C9, or CYP2C19 substrates, if clinically indicated. If co-administration with warfarin (a CYP2C9 substrate) cannot be avoided, consider increasing frequency of international normalized ratios (INR) monitoring.
Because of the potential for serious adverse reactions in breast-fed children from Rubraca, advise lactating women not to breastfeed during treatment with Rubraca and for 2 weeks after the last dose.
You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Clovis Oncology, Inc. at 1-844-258-7662.
Please see U.S. Prescribing Information for additional Important Safety Information.
About Clovis Oncology
Clovis Oncology, Inc. is a biopharmaceutical company focused on acquiring, developing and commercializing innovative anti-cancer agents in the U.S., Europe and additional international markets. Clovis Oncology targets development programs at specific subsets of cancer populations, and simultaneously develops, with partners, diagnostic tools intended to direct a compound in development to the population that is most likely to benefit from its use. Clovis Oncology is headquartered in Boulder, Colorado, and has additional offices in San Francisco, California and Cambridge, UK. Please visit clovisoncology.com for more information.




