Recce Pharmaceuticals Ltd, the Company developing a new class of synthetic anti-infectives, announced positive efficacy activity against Helicobacter pylori bacteria in rats treated with new antibiotic RECCE® 435, including a favorable toxicity profile in a related study.
Highlights:
- New RECCE® 435 oral showed dose-dependent and efficacy against Helicobacter pylori (H. pylori) bacteria isolated from a patient with a duodenal ulcer compared to control vehicle in independent study model in rats
- Separate and independent repeat oral dosing study indicates 500mg/kg twice daily vs. water control yielded no observed toxicity with favorable weight gain throughout
- High solubility and antibacterial effect supportive of a ‘targeted’ oral therapy for stomach infection
- Discussions with world leading H. pylori experts to assess commercial pathway
SYDNEY, Australia, Aug. 05, 2020 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Ltd (ASX: RCE) (Company), the Company developing a new class of synthetic anti-infectives, today announced positive efficacy activity against Helicobacter pylori (H. pylori) bacteria in rats treated with new antibiotic RECCE® 435, including a favorable toxicity profile in a related study. RECCE® 435 is a broad-spectrum synthetic polymer antibiotic formulated for oral use.
“The Company is most encouraged by these data, further indicating a long anticipated potential against H. pylori, a significant pathogen with a particular prevalence in the neighboring Asia-Pacific region,” said Dr. John Prendergast, Recce Pharmaceuticals Non-Executive Chairman. “This study further endorses our ever promising therapeutic potential to advance a new class of synthetic antibiotics and anti-infectives for the treatment of a wide spectrum of pathogens capable of causing deadly infections. While Recce is pleased with these results, they do not mean that the RECCE® 435 compound will be safe or effective for use in humans.”
The efficacy study was conducted by an independent Contract Research Organization to assess oral dose-dependent efficacy of RECCE® 435 in-vivo (rats) against a clinical isolate of H. pylori. H. pylori is a species of Gram-negative bacteria commonly infecting the lining of the stomach and upper digestive tract. There is no available first-line therapy that is curative in all patients at this time and it is a major cause of morbidity and mortality worldwide; it is estimated more than 50% of the global population is infected.1,2
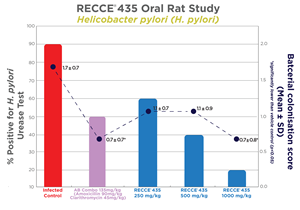
In the study, five groups of 10 rats each were observed. Three of these groups were treated with varying doses of RECCE® 435 (250, 500, 1,000 mg/kg) and dose-dependent efficacy was seen at all doses with significant reduction in bacterial load. Upon completion of the study, a urease test was carried out upon the stomach lining to confirm the presence of H. pylori in the subjects. Helicobacter pylori can survive within the acidic environment of the stomach by producing an enzyme called urease. Therefore, H. pylori presence was measured by a urease diagnostic test in the stomachs of rats in the study with those. No signs of toxicity were observed at any dosage level throughout the efficacy study.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/2e899f19-b3ca-4432-a304-9000d38b5c13
| Group | Group ID | Rats | Urease test | % Positive for H. pylori [Urease Test] | |
| Positive | Negative | ||||
| 1 | Uninfected control | 10 | 0 | 10 | 0 |
| 2 | Infected control | 10 | 9 | 1 | 90 |
| 3 | AB Combo 135 mg/kg (Amoxicillin 90 mg/kg + Clarithromycin 45 mg/kg) | 10 | 5 | 5 | 50 |
| 4 | Infected + RECCE® 435 - 250 mg/kg | 10 | 6 | 4 | 60 |
| 5 | Infected + RECCE® 435 - 500 mg/kg | 10 | 4 | 6 | 40 |
| 6 | Infected + RECCE® 435 - 1000 mg/kg | 10 | 2 | 8 | 20 |
RECCE® 435 mg/kg dosing is based upon ‘total administered solution’. A significant proportion of RECCE® 435 administered solution quoted includes inactive components such as diluent/water and stabilizing medium. The Active Pharmaceutical Ingredient (API) as is sometimes the quoted mg/kg of the comparative product/s, likely to dramatically benefit by way of reduction to the otherwise stated RECCE® figure.
This study assessed a combination of two broad spectrum antibiotics being used – Amoxicillin and Clarithromycin. Amoxicillin was used at a higher dosage, being one of the most active antimicrobials against H. pylori.3 This standard therapy has recently been undermined by its ineffectiveness for a number of reasons including the development of high resistance rates and the lack of novel drugs.
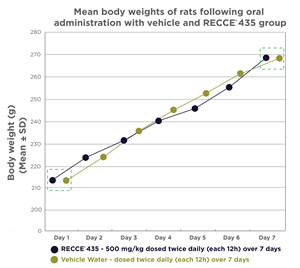
An additional independent study examining the safety of oral dosing of RECCE® 435 up to 500mg/kg was administered to groups of five mice each twice daily for seven days, compared to water-only administration. The data indicates their feeding habits, which contributes to weight gain, were not negatively impacted, supporting overall general and gastrointestinal health.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c1a9300a-c2e6-42a9-98ed-3c7e276d7300
| Mean body weights of rats following oral administration with vehicle and RECCE® 435 group | Body weight (g) (Mean ± SD) | ||||||
| Days | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
| Vehicle Water – dosed twice daily (each 12h) over 7 days | 213 ± 8.09 | 224.4 ± 6.73 | 236.2 ± 4.82 | 246 ± 5.15 | 253.2 ± 4.15 | 262.6 ± 3.65 | 268.2 ± 5.81 |
| RECCE® 435 - 500 mg/kg dosed twice daily (each 12h) over 7 days | 213.4 ± 4.56 | 223.4 ± 9.32 | 231.6 ± 7.7 | 240 ± 4.74 | 246.8 ± 5.89 | 255.2 ± 9.65 | 269.4 ± 5.77 |
H. pylori was recently added by the FDA to the Agency's list of qualifying pathogens that have the potential to pose a serious threat to public health. As a result, drug treatments being studied for patients with H. pylori infection have been granted Qualified Infectious Disease Product (QIDP) designation. In addition to H. pylori increasing risk of ulcers and other gastric diseases, research suggests that some 35-60% of gastric adenocarcinomas are attributable to H. pylori infection.4
The World Health Organisation (WHO) also lists H. pylori as a priority pathogen on its list of antibiotic-resistant bacteria that pose the greatest threat to human health.5Helicobacter pylori is known to cause stomach inflammation (gastritis) and more serious conditions such as stomach ulcers and stomach cancer.6
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE) is pioneering the development and commercialization of new classes of synthetic anti-infectives designed to address the urgent global health problems of antibiotic resistant superbugs and emerging viral pathogens.
Recce antibiotics are unique – their potency does not diminish even with repeated use, a common failure associated with existing antibiotics and their propensity to rapidly succumb to resistant superbugs.
Patented lead candidate RECCE® 327, wholly owned and manufactured in Australia, has been developed for the treatment of blood infections and sepsis derived from E. coli and S. aureus bacteria – including their superbug forms.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval.
Recce wholly owns its automated manufacturing, ready to support first-in-human clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of RECCE® technologies targeting synergistic, unmet medical needs.
1https://www.mja.com.au/journal/2016/204/10/epidemiology-clinical-impacts-and-current-clinical-management-helicobacter
2https://www.racgp.org.au/afp/2014/may/helicobacter-pylori-eradication/
3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5314729/
4https://journals.lww.com/ctg/Abstract/2013/03000/Helicobacter_Pylori_Test_and_Treat__Strategy_for.2.aspx
5WHO list of bacteria
6ttps://www.healthdirect.gov.au/helicobacter-pylori
| Executive Director | Media & Investor Relations (AU) | Media & Investor Relations (USA) |
| James Graham | Andrew Geddes | Meredith Sosulski, PhD |
| Reece Pharmaceuticals, Ltd. | CityPR | LifeSci Communications |
| +61 (02) 8075 4585 | +61 (02) 9267 4511 | +1 929 469 3851 |
| james.graham@reece.com.au | ageddes@citypublicrelations.com.au | msosulski@lifescicomms.com |