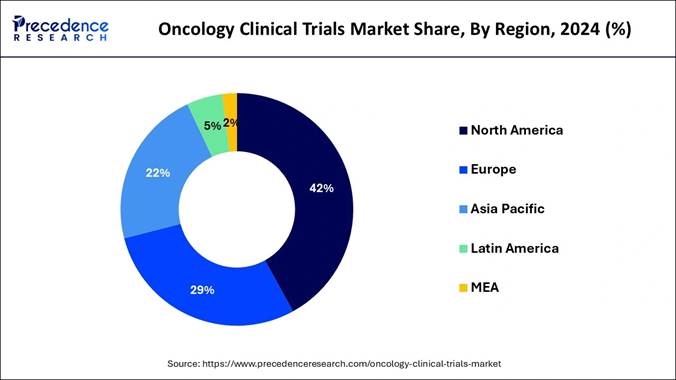
The global oncology clinical trials market size is calculated at USD 14.27 billion in 2025 and is predicted to grow from USD 14.98 billion in 2026 to approximately USD 22.76 billion by 2034, growing at a CAGR of 5.28% from 2025 to 2034.
Oncology Clinical Trials Market
Key Takeaways 🔹 The global oncology clinical trials market
size reached USD 13.60 billion in 2024 and is set to climb to USD
22.76 billion by 2034. 🔹 The industry is projected to grow at a CAGR
of 5.28% from 2025 to 2034, driven by rising cancer incidence and expanded
trial pipelines. 🔹 North America led the market in 2024,
accounting for over 42% of global revenue, supported by strong R&D
infrastructure and high clinical trial activity. 🔹 Asia-Pacific is poised to grow at the fastest
CAGR, fueled by expanding trial networks, supportive regulations, and lower
operating costs. 🔹 By phase type, Phase III trials dominated
with over 49% revenue share in 2024, reflecting the high volume and cost of
late-stage studies. 🔹 Phase I trials are expected to witness a
strong CAGR of 5.14%, driven by the surge in early-stage oncology
innovations and targeted therapies. 🔹 By study design, interventional studies
accounted for more than 88% of revenue in 2024, emphasizing their
critical role in evaluating cancer treatments. 🔹 Observational studies are projected to
record the fastest CAGR, supported by growing real-world evidence (RWE)
adoption and post-marketing surveillance needs. Oncology Clinical Trials Market
Overview Oncology clinical trials are investigative
studies that are designed to explore innovative treatments and interventions
for individuals who are diagnosed with cancer. These trials aim to evaluate the
safety and effectiveness of emerging modalities, including pharmaceuticals,
immunotherapies and targeted treatments. The main goal of this market is advancing
cancer therapeutics. The oncology clinical
trials market is rapidly evolving, driven by
technological advancements and more patient-centric approaches. Regulatory
bodies like the FDA have streamlined processes to propel trial approvals for
breakthrough therapies, thus encouraging a more efficient pathway. The
application of oncology clinical trials spans across multiple industries,
including pharmaceuticals, biotechnology and academics. Collaborative efforts between
pharmaceutical companies and research institutions are also seen becoming
common, fostering innovation and enhancing the development of new therapies.
Additionally, the integration
of digital health technologies, such as telemedicine and mobile health
applications, is transforming how trials are
conducted and how patients engage with their care. ➡️ Become a valued
research partner with us ☎
https://www.precedenceresearch.com/schedule-meeting What are the Major Drifts in
Oncology Clinical Trials Market? Advancements in biotechnology are increasingly
revolutionizing the market. Innovations such as gene therapy, monoclonal
antibodies and CAR-T
cell therapy are seen optimizing cancer
treatment paradigms. The biotechnology sector has also seen a surge in
investment, with funding for cancer-related biotech startups reaching high levels.
🔸Globally,
between 1999 and 2023, there have been 112,899
clinical trials targeting cancer (across all types of interventions). This influx of capital is
facilitating the development of novel therapies, pushing companies to
experiment and innovate, which in turn, drives the need for advanced clinical
trials. Remote monitoring, telemedicine, and data
analytics are becoming vital components of trial designs, as they allow for more efficient patient recruitment and
retention. This not only improves the overall patient experience but also
enhances the quality of data that is collected during trials. Role of AI in Oncology Clinical
Trials Market: AI
is increasingly becoming foundational in oncology clinical trials,
enabling smarter trial design, faster and more accurate patient matching, and
more efficient data analysis. By leveraging machine‑learning
and deep‑learning
tools, AI can sift through large, complex datasets (electronic
health records, imaging, genomics,
biomarker data, unstructured clinical notes) to identify patients who meet
trial eligibility criteria and are most likely to benefit reducing the often
long, costly, and inefficient process of patient recruitment. 🔸AI
also helps optimize trial protocols (e.g. selecting relevant endpoints,
stratifying based on tumor biomarkers, tailoring dose regimens) and improves
monitoring / data‑management during the trial (handling imaging,
histopathology, molecular data, adverse events). Unlock detailed insights on AI’s impact in the Oncology Clinical Trials
market 👉 https://www.precedenceresearch.com/ai-precedence Major Government Initiatives
for Oncology Clinical Trials Sector: Initiative / Programme Country / Region Key Features / Relevance to Oncology Clinical
Trials National Cancer Grid (NCG) India Network of cancer centers; sets uniform standards;
supports multicenter clinical trials. Ayushman Bharat – PMJAY India Provides subsidized cancer treatment; facilitates
patient access for trials. National Cancer Institute (NCI) Clinical Trials
Program USA Funds and organizes large-scale cancer trials;
supports early and late-phase studies. Cancer Grand Challenges Global (US-UK) Funds high-risk, innovative cancer research feeding
into new clinical trials. NIHR Research Delivery Network & Innovation
Funding UK Provides infrastructure and funding for cancer
clinical trials and early diagnostics. Medical Research Council (MRC) UK Funds translational and clinical cancer research;
supports non-commercial trials.
What are the Key Trends in the Oncology
Clinical Trials Market? 🔹Shift Toward Decentralized Clinical Trials (DCTs): Oncology
trials are seen increasingly adopting remote monitoring, telemedicine, and home
health services in order to enhance patient convenience and recruitment
diversity. 🔹Expansion of Biomarker-Driven and Genotype-Based Trials:
Trials are now designed around specific genetic mutations, which allows for
targeted therapy development, especially in cases of lung, breast and
colorectal types of cancers. 🔹Integration of Artificial Intelligence: AI and machine
learning tools are increasingly being integrated in order to identify eligible
patients, optimize site selection, predict trial outcomes and analyze imaging
or even genomic data. 🔹Adaptive Trial Designs: The market is witnessing a rise in
adaptive trial designs which basically allows modifications to trial parameters
mid-study, thus improving efficiency and reducing the waste of resources. 🔹Growing Collaboration Between Pharma and CROs: Several pharmaceutical
companies all over the world are partnering up with specialized CROs for trial
design, site management and regulatory compliance. This helps to reduce costs
and streamline trial operations. 📥
Dive into the Complete Report ➡️
https://www.precedenceresearch.com/oncology-clinical-trials-market What are the Major Challenges
faced by the Market? Despite the promising growth of
the oncology clinical trial market, the market does have several restraints that
could hinder its growth and development. One such significant challenge is the
complex regulatory landscape surrounding clinical trials. Regulatory bodies,
such as the U.S. FDA and the European Medicines Agency (EMA), impose stringent
guidelines in order to ensure patient safety and data integrity. While these
regulations are quite vital, they can also lead to long, time-consuming approval
processes that delay trial initiation and also increase costs. This can create
financial challenges for many organizations and companies. Another restraint is the patient
recruitment and reluctancy. Many potential patients are often reluctant to
participate due to concerns regarding side effects, the burden of additional
medical visits or a lack of awareness about available trials. These factors can
lead to delays and ultimately slow down market entry and potential. Oncology Clinical Trials Market
Report Coverage Report Coverage Details Base Year 2024 Market Size (2025) USD 14.27 Billion Market Size (2026) USD 14.98 Billion Forecast Market Size (2034) USD 22.76 Billion Forecast Period 2025 to 2034 CAGR (2025–2034) 5.28% Segments Covered Phase Type (Phase I, II, III, IV); Study
Design (Interventional, Observational, Expanded Access) Regions Covered North America, Europe, Asia-Pacific, Latin
America, Middle East & Africa Leading Region (2024) North America (~42% share) Fastest-Growing Region Asia-Pacific Dominant Phase (2024) Phase III (49%+ revenue share) Fastest-Growing Phase Phase I (CAGR ~5.14%) Dominant Study Design (2024) Interventional Studies (88%+ share) Fastest-Growing Study Design Observational Studies Key Market Drivers Rising global cancer cases; surge in targeted
& personalized therapies; growing R&D investments; increasing
clinical trial activity Key Market Restraints High trial costs; complex regulatory
frameworks; patient recruitment & retention challenges Emerging Opportunities Decentralized trials, AI-driven recruitment,
biomarker-based studies, digital monitoring tools, real-world evidence
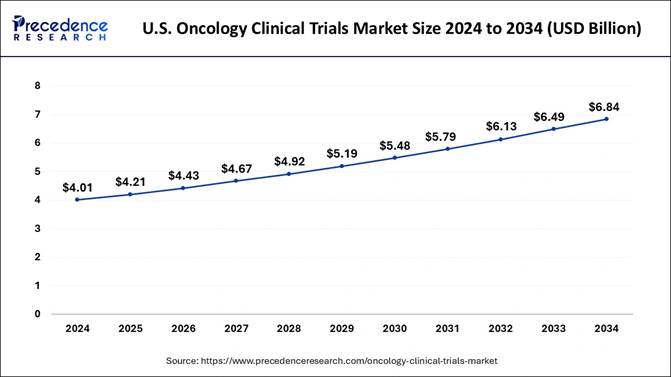
integration U.S. Market Insight Expected to grow from approx. USD 4.01 Billion
(2024) to USD 6.84 Billion (2034) at ~5.49% CAGR
Don’t Miss Out! | Instant
Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/3672 Oncology Clinical Trials Market
Regional Outlook North America Leads the Global Oncology Trial Ecosystem North
America continues to dominate the global oncology clinical
trials market, contributing the largest share of trial activity and revenue.
Its leadership is supported by advanced healthcare systems, strong research
funding, cutting-edge cancer institutes, and highly experienced trial networks. The
region benefits from an innovation-friendly environment, early adoption of new cancer therapeutics, and
significant collaboration between academic centers, biotechnology companies,
pharmaceutical giants, and clinical research organizations. Despite rising
operational costs and increasing pressure for diverse patient inclusion, North
America remains the benchmark for oncology trial excellence. How Big is the Oncology Clinical Trials Market?
According to Precedence Research, the U.S. oncology clinical trials market
size is expected to reach nearly USD 6.84 billion by 2034, up from USD 4.21
billion in 2025, growing at a CAGR of 5.49% from 2025 to 2034. The
United States holds the highest concentration of oncology clinical trials
globally. This position is driven by high R&D investments, advanced genomic
and biomarker infrastructure, and a mature regulatory environment that supports
accelerated approval pathways for cancer therapies. Thousands
of ongoing trials across immuno-oncology, targeted therapy, radiopharmaceuticals, and precision medicine underscore the
country's unmatched leadership in cancer research and clinical development. Note: This report is
readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for
decision-making. Asia Pacific Fastest Growing Region in Oncology Trials Asia
Pacific is the fastest growing region in the oncology clinical trials market.
Countries such as China, Japan, South Korea, and Australia are rapidly
expanding their trial capabilities with improved healthcare infrastructure,
growing investments in oncology research, and large patient populations that enable
faster recruitment. The
region is increasingly preferred for precision oncology trials due to cost
efficiency, diverse patient representation, and accelerating regulatory reforms
that support high-quality clinical research. India Emerging as a High Potential Oncology Trial Destination India
is steadily gaining recognition as a promising market for oncology clinical
trials. The country offers a large and diverse patient pool, cost-effective
trial execution, and an expanding network of oncology-focused hospitals and
research centers. Although
regulatory and logistical challenges persist, India is improving its clinical
research ecosystem with better oversight, growing oncology specialization, and
increased collaboration with global sponsors. This positions India to become a
significantly more competitive player in the global oncology trial landscape. Oncology Clinical Trials Market
Segmental Insights Phase Type Insights Which phase type dominated the
market in 2025? The Phase 3 trials segment dominated
the market in 2025. This dominance is because they provide all the essential
data that is required for the regulatory approval of new therapies. This
segment usually involves larger populations and longer durations, which helps to
establish the efficacy and monitoring of adverse reactions in diverse patient
demographics. These trials analyze the effectiveness and safety of treatments
on a larger scale, and often involves thousands of patients. The Phase 1 trials segment is
expected to have the fastest growth rate during the forecast years. This
segment represents the emerging front in clinical research, and focuses
primarily on the safety and dosage in a small group of participants. This phase
is seen attracting interest for novel treatment methodologies and increased
funding, as they lay the groundwork for subsequent phases. Study Design Insights Which study design led the
market as of this year? The
interventional studies segment led the market as of this year. This segment actively analyzes the impact of specific
treatments on cancer outcomes. They are carried out through randomized
controlled trials, and they evaluate the safety and effectiveness of emerging
therapies or different treatment combinations. The shift towards personalized
medicine and customized interventions is expected to propel the segment forward
even more. The
observational studies segment is anticipated to grow the fastest over the
forecast period. This segment involves the
systematic collection and analysis of real-world data in order to understand
the natural history of diseases, treatment outcomes and patient
characteristics. These studies aim to observe participants in their everyday
settings, providing valuable insights into the effectiveness and safety of
cancer treatments outside controlled trial environments. ✚ Related
Topics You May Find Useful: ➡️ Virtual Clinical Trials Market: Explore how decentralized
technologies are reshaping patient participation and accelerating study
timelines ➡️ Cell and Gene Therapy Clinical Trials Market: Track rapid advancements
in next-gen therapeutics and global expansion of CGT trial ecosystems ➡️ Clinical Trial Patient Recruitment Services Market: Discover how digital
outreach and AI-driven screening are transforming patient enrollment ➡️ Cancer Drugs
Market:
Analyze
the surge in immunotherapy, targeted treatments, and precision oncology
adoption worldwide ➡️ Oncology Molecular Diagnostic Market: See how biomarker
testing and genomic profiling are boosting personalized cancer care ➡️ Cancer Biological Therapy Market: Understand how biologics and
immune-modulating therapies are redefining cancer treatment paradigms ➡️ AI-Based Clinical Trials Solution Provider Market: Gain insights into how
AI is revolutionizing trial design, patient matching, and data analytics Oncology Clinical Trials Market
Top Companies ➢ Novartis ➢ Merck & Co. ➢ Pfizer Inc. ➢ Roche ➢ Bristol Myers Squibb ➢ AstraZeneca ➢ Johnson & Johnson ➢ Eli Lilly and Company ➢ GlaxoSmithKline ➢ Sanofi ➢ AbbVie Inc. ➢ Celgene Corporation (now part of Bristol Myers Squibb) ➢ Astellas Pharma Inc. ➢ Daiichi Sankyo ➢ Takeda Pharmaceutical Company Limited Recent Developments 🔸 In December 2025, Aurigene Oncology engaged in developing
novel therapies in oncology, and has yielded positive initial clinical results
from 1st two cohorts of its ongoing Phase 1 clinical trial of the drug
candidate, AUR112, in patients with relapsed or refractory lymphoid
malignancies. The early findings show that AUR112 is “safe, well-tolerated,
demonstrating meaningful clinical activity, with objective responses observed
across multiple lymphoma subtypes. (Source: https://www.thehindubusinessline.com) 🔸In December 2025, BetaGlue Therapeutics, a pioneering
Italian clinical-stage oncology company developing an innovative radiotherapy
solution for the targeted treatment of solid tumors, recently announced that
the Italian Ministry of Health (MOH) has approved its Clinical Trial
Application for YntraDose in the treatment of unresectable pancreatic cancer.
This approach has been designed with the aim to maximize therapeutic efficacy
while minimizing damage to surrounding healthy tissue, offering new hope for
patients. (Source: https://www.manilatimes.net) Segments Covered in the Report By Phase Type 🔹 Phase I 🔹 Phase II 🔹 Phase III 🔹 Phase IV By Study Design 🔹 Interventional Studies 🔹 Observational Studies 🔹 Expanded Access Studies By Region 🔹
North America 🔹
Europe 🔹
Asia Pacific 🔹Latin
America 🔹Middle
East & Africa (MEA) Thanks for reading you can
also get individual chapter-wise sections or region-wise report versions such
as North America, Europe, or Asia Pacific. Don’t Miss Out! | Instant Access to
This Exclusive Report 👉
https://www.precedenceresearch.com/checkout/3672 You can place an order or ask any
questions, please feel free to contact at sales@precedenceresearch.com
| +1 804 441 9344 Stay Ahead with Precedence
Research Subscriptions Unlock exclusive access to powerful market intelligence, real-time
data, and forward-looking insights, tailored to your business. From trend
tracking to competitive analysis, our subscription plans keep you informed,
agile, and ahead of the curve. Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription About Us Precedence Research is a global market intelligence and
consulting powerhouse, dedicated to unlocking deep strategic insights that
drive innovation and transformation. With a laser focus on
the dynamic world of life sciences, we specialize in decoding
the complexities of cell and gene therapy, drug development, and oncology markets,
helping our clients stay ahead in some of the most cutting-edge and high-stakes
domains in healthcare. Our expertise spans across the biotech and
pharmaceutical ecosystem, serving innovators, investors, and institutions that
are redefining what’s possible in regenerative
medicine, cancer care, precision therapeutics, and beyond. Web: https://www.precedenceresearch.com ✚ Explore More Market Intelligence from Precedence Research: ➡️ Generative AI in Life Sciences: Explore how AI innovations are
revolutionizing drug discovery, research efficiency, and precision medicine. ➡️ Biopharmaceuticals Growth: Understand the accelerating expansion
of biologics, therapeutic proteins, and cutting-edge pharma pipelines. ➡️ Digital Therapeutics: Discover how technology-driven
treatments are reshaping patient care and improving clinical outcomes. ➡️ Life Sciences Growth: Gain insights into emerging
opportunities, market expansion, and innovation trends in the life sciences
sector. ➡️ Viral Vector & Gene Therapy Manufacturing: Analyze the production advancements
powering next-generation gene therapies and precision medicine. ➡️ Wellness Transformation: See how consumer wellness trends are
shaping supplements, functional foods, and lifestyle-driven markets. ➡️ Generative AI in Healthcare: Unlocking Novel
Innovations in Medical and Patient Care: Explore AI applications enhancing diagnostics, treatment
personalization, and patient engagement. Our Trusted Data Partners: Towards Healthcare | Nova One
Advisor | Onco Quant | Statifacts Get Recent News 👉
https://www.precedenceresearch.com/news For Latest Update Follow Us: LinkedIn
| Medium | Facebook | Twitter 
The Complete Study is Now Available for Immediate Access | Download the Sample
Pages of this Report@ https://www.precedenceresearch.com/sample/3672
🔸In
2023 alone, more than 2,000
new oncology clinical trials were initiated worldwide many of
these trials explore novel modalities such as cell and gene therapies,
antibody-drug conjugates (ADCs), multispecific antibodies, radioligand
therapies, etc.
f
United States Spotlight the World's Largest Oncology Trial Hub
📥 Download
Sample Pages for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/3672