SINGAPORE, Dec. 5, 2025 — NeuExcell Therapeutics announced encouraging clinical results of NXL-004, the world’s first in situ conversion gene therapy for the treatment of malignant glioma. The data were presented at the ESMO Asia Congress 2025, December 5–7 in Singapore.
Recurrent malignant glioma carries an extremely poor prognosis, with a median survival of less than nine months. NXL-004 is the first-in-class gene therapy utilizing an adeno-associated virus (AAV) vector to deliver neural transcription factor NeuroD1 directly into glioma cells and reprogram tumor cells into non-dividing neurons or induce apoptosis, representing a novel therapeutic paradigm.
This first-in-human clinical study was designed to evaluate the safety and efficacy of intracranial administration of NXL-004 in patients with recurrent malignant glioma. The trial was jointly conducted by the Fourth Affiliated Hospital of Soochow University and NeuExcell Therapeutics.
A total of 11 patients with recurrent malignant glioma following surgery and chemoradiotherapy were enrolled. Among them, 10 patients received surgical tumor resection followed by intracavitary injection of NXL-004, and one patient received intratumoral NXL-004 injection following biopsy.
Key study findings include:
· Safety: NXL-004 demonstrated a favorable safety profile. No drug-related serious adverse events or dose-limiting toxicities were observed.
· Efficacy: After treatment with NXL-004, the estimated median overall survival (OS) for all patients exceeded 12 months, significantly surpassing historical control data of 6–9 months. The first enrolled patient survived over 18 months following surgery and NXL-004 treatment.
· Tumor Response: In the patient receiving intratumoral injection, a clear therapeutic response was observed, with target lesion shrinkage exceeding 90%.
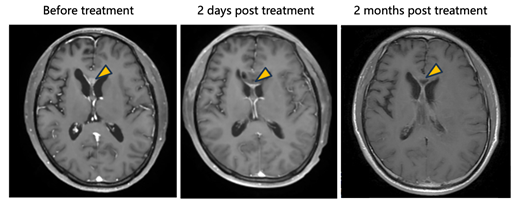
· In one patient treated with tumor resection followed by intracavitary injection of NXL-004, postoperative imaging demonstrated progressive tumor reduction leading to complete radiographic disappearance, achieving a complete response (CR).
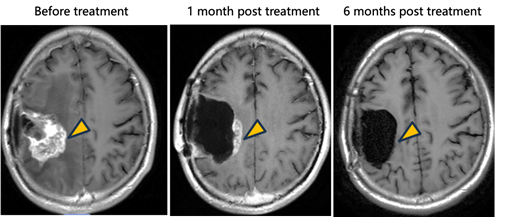
The results demonstrate that NXL-004 exhibits both a favorable safety profile and promising clinical efficacy, validating the feasibility and therapeutic potential of in situ conversion therapy in the treatment of glioma.
About ESMO Asia:
ESMO Asia is the premier oncology conference in the Asia-Pacific region, convening global experts to disseminate breakthrough research and clinical innovations.
About NeuExcell Therapeutics:
NeuExcell is a clinical-stage biotech company focusing on the development of innovative treatments for neurological diseases. NeuExcell has developed a core platform to reprogram internal glial cells directly into functional neurons via in situ conversion approach, targeting a variety of neurological disorders including glioma, Alzheimer’s, Parkinson’s, and stroke.
Contact: info@neuexcell.com




