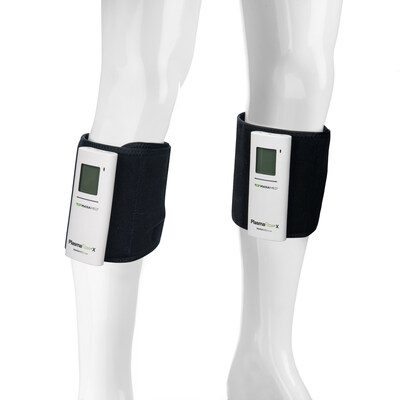
Over 30% smaller footprint, double the battery life, modern USB – C charging, and an expanded LCD interface set a new bar for performance and portability in SCDs.
DENTON, Texas, Oct. 20, 2025 /PRNewswire/ -- ManaMed today announced FDA 510(k) clearance for PlasmaFlow® X, the next-generation, tubeless sequential compression system that's redefining recovery. Cleared as a Class II device, PlasmaFlow X is more compact, smarter, and longer-lasting—delivering powerful therapy in a sleek, portable package.
Designed from the inside out, PlasmaFlow® X delivers a compact design to help save shelf space in surgery centers and warehouses nationwide—while reducing packaging waste. With an all–day battery that offers double the battery life vs. the previous PlasmaFlow model, the latest controller architecture, and USB–C charging, it's built for seamless, everyday use at home and across care settings. An expanded LCD presents the essentials instantly—battery charge status, pressure (mmHg), patient usage, and adherence—bringing a modern, intuitive experience to sequential compression. The low–profile, lightweight sleeve is engineered for comfort and a sleek fit for patients on the move.
"PlasmaFlow X is a generational leap for sequential compression," said Trevor Theriot, President + CEO of ManaMed. "In a category long defined by incremental updates, our team re–imagined everything—a more compact design, all–day battery, USB–C convenience, and a big beautiful, informative display—to deliver industry leading therapy in a remarkably portable system. This is our most exciting product launch in ManaMed's 10–year history, and it underscores our commitment to innovation that serves patients, clinicians, and partners."
Regulatory & intended use
PlasmaFlow X is cleared via 510(k) as a compressible limb sleeve device for aiding in DVT prevention, can be used as an aid in the prophylaxis for DVT by persons expecting to be stationary for long periods of time, diminish post-operative pain and swelling, and enhancing blood circulation. The device features preset compression and a streamlined, tubeless configuration to support consistent therapy in clinical and home environments. For complete labeling and indications for use, please refer to the FDA 510(k) and the product's instructions for use.
Availability
PlasmaFlow X is now cleared for marketing in the United States and will be available through ManaMed and authorized distributors. For ordering and specifications visit ManaMed.com or email support@manamed.com.
About ManaMed
ManaMed designs, develops, and distributes orthopedic solutions that keep active, high-performing people in the game and back-on-track following injury or surgery. Our extensive catalog spans vascular and electrotherapies, assisted mobility, electrical stimulation, and DVT prevention products. We partner with orthopedists, physical therapists, surgeons, and DME companies across the US to keep patients of all ages and backgrounds healthy and comfortable while on the path to recovery. We welcome opportunities to work together to solve tough challenges with innovative solutions. For more information, visit manamed.com, or talk to one of us by emailing support@manamed.com or calling 1.888.508.0712.
Media Contact:
Stephanie Green
Sgreen@ManaMed.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/manamed-secures-fda-510k-clearance-for-plasmaflow-x--a-breakthrough-compact-sequential-compression-system-with-allday-battery-302589462.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/manamed-secures-fda-510k-clearance-for-plasmaflow-x--a-breakthrough-compact-sequential-compression-system-with-allday-battery-302589462.html
SOURCE ManaMed LLC