According to Coherent Market Insights, Hereditary Angioedema Market size is estimated to be valued at USD 3.13 Bn in 2025 and is expected to reach USD 5.96 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.6% from 2025 to 2032. The growth of the Hereditary Angioedema Market is primarily driven by the rising awareness of the disorder and the introduction of advanced treatment options. The development of improved diagnostic tools has enabled earlier and more accurate detection of the condition, leading to an expanding patient base and a growing demand for effective therapies.
Request Sample Report: https://www.coherentmarketinsights.com/insight/request-sample/1292
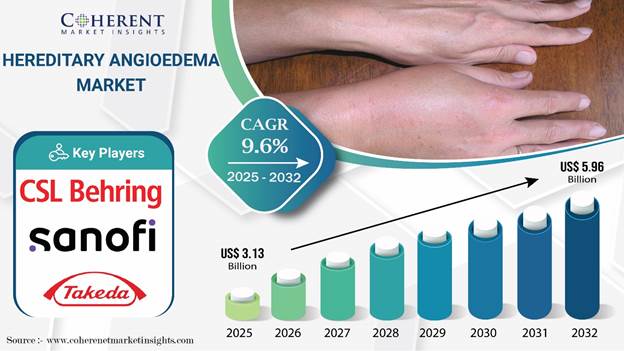
Global Hereditary Angioedema Market Key Takeaways
Hereditary angioedema type I is expected to dominate the market, capturing a revenue share of 67.8% in 2025.
C1 esterase inhibitors remain the most popular drug class for treating hereditary angioedema, accounting for 30.1% of the market share in 2025.
Based on route of administration, subcutaneous segment is set to account for 30.1% of the global hereditary angioedema market share by 2025.
North America, with an estimated share of 36.3% in 2025, is expected to lead the global hereditary angioedema industry.
Asia Pacific is poised to emerge as the most lucrative pocket for hereditary angioedema market companies throughout the forecast period.
Increasing Diagnosis and Awareness Fueling Market Growth
Coherent Market Insights’ latest hereditary angioedema market analysis outlines key factors driving industry growth. One such prominent growth driver is the increasing diagnosis and awareness of hereditary angioedema (HAE).
There is a growing awareness of hereditary angioedema among healthcare professionals and general public. This is leading to more diagnoses, thereby creating a conducive environment for the growth of hereditary angioedema market.
Improvements in diagnostic technologies like genetic testing and more precise clinical tools are helping in earlier and more accurate identification of HAE cases. These trends are expected to increase demand for HAE therapies over the forecast period.
Also Read: Angioedema Treatment Market Size, Share, Trends & Opportunities for 2025-2032
High Treatment Costs and Delayed Diagnosis Limiting Market Growth
The global hereditary angioedema market outlook appears promising, owing to improvements in disease diagnosis. However, high treatment costs and delayed diagnosis in some regions might limit market growth to some extent during the forthcoming period.
Hereditary angioedema therapies, especially biologics and prophylactic therapies, are quite expensive. This may limit their adoption during the forecast period, thereby dampening overall hereditary angioedema market demand.
In addition, many HAE patients are misdiagnosed because it is a rare disease with symptoms that resemble common conditions like allergies or stomach problems. This often delays proper diagnosis for years, leading to late treatment and slowing hereditary angioedema market growth.
Advancements in Treatment Options Unlocking Growth Opportunities
Leading companies are striving to develop novel therapies like biologics, monoclonal antibodies, and kallikrein inhibitors to prevent HAE attacks. For instance, KalVista Pharmaceuticals’ novel plasma kallikrein inhibitor (EKTERLY) recently got approval from the U.S. FDA for the treatment of acute attacks of HAE in adult and pediatric patients aged 12 and older. Introduction of these new treatments is expected to create lucrative growth opportunities for the HAE market.
There is also a growing shift towards more patient-friendly administration routes like oral therapies and subcutaneous injections. These innovations significantly improve convenience as well as patient adherence.
Immediate Delivery Available | Buy This Premium Research Report: https://www.coherentmarketinsights.com/insight/buy-now/1292
Emerging Hereditary Angioedema Market Trends
Shift towards prophylactic treatments is a key growth-shaping trend in the hereditary angioedema market. There is an increasing demand for long-term prophylactic treatments to reduce frequency as well as severity of HAE attacks. This trend is expected to boost sales of hereditary angioedema drugs during the forecast period.
Oral on-demand treatments like KalVista’s Ekterly are gaining traction in hereditary angioedema management. This is due to their convenience, rapid onset of action, and ease of use compared to traditional injectable therapies. Increasing usage of these oral on-demand treatments is expected to boost hereditary angioedema market value during the forthcoming period.
Regulatory agencies like the FDA and EMA are helping the industry by approving new treatments quickly. For example, the FDA recently approved DAWNZERA (donidalorsen) as the first and only RNA-targeted preventive treatment for HAE. These approvals allow effective HAE treatments to reach patients faster.
Also Read: Hereditary Testing Market Analysis & Forecast for 2025-2032
Analyst’s View
“The global hereditary angioedema industry is set for rapid growth, owing to rising awareness and diagnosis of HAE, increasing regulatory support, growing popularity of oral on-demand HAE treatments, and advancement in treatment options,” said a senior CMI analyst.
Current Events and Their Impact on the Hereditary Angioedema Market
|
Event |
Description and Impact |
|
FDA Regulatory Modernization and Orphan Drug Incentives |
|
|
Breakthrough Gene Therapy and CRISPR Technologies |
|
|
Healthcare Digitalization and Telemedicine Adoption |
|
Competitor Insights Key companies in hereditary
angioedema market report: - Sanofi - CSL Behring - Orchard Therapeutics plc. - Ionis Pharmaceuticals,
Inc. - Pharming Group N.V. - BioCryst Pharmaceuticals,
Inc. - Takeda Pharmaceutical
Company Limited - Arrowhead Pharmaceuticals,
Inc. - Attune Pharmaceuticals - Adverum Biotechnologies,
Inc. - CENTOGENE N.V. - KalVista Pharmaceuticals Key Developments ·
In
August 2025, the U.S. FDA approved Ionis Pharmaceuticals’ DAWNZERA (donidalorsen) as a
prophylactic treatment to prevent hereditary angioedema attacks in patients
aged 12 years and older. This comes after DAWNZERA showed significant HAE
attack rate reduction during clinical trials. DAWNZERA becomes the first and
only RNA-targeted therapy approved for prophylaxis in HAE. ·
In
July 2025, the U.S. FDA approved KalVista Pharmaceuticals’ EKTERLY (sebetralstat)
for the treatment of acute attacks of HAE in adult and pediatric patients aged
12 and older. This novel plasma kallikrein inhibitor (EKTERLY) is the first and
only oral on-demand treatment for hereditary angioedema. Request For Customization: https://www.coherentmarketinsights.com/insight/request-customization/1292 Market Segmentation Also Read: Hereditary Spastic
Paraplegia Market Outlook for 2025-2032 Our Trusted Partners: Worldwide Market Reports, Coherent MI, Stratagem Market Insights Get Recent News: https://www.coherentmarketinsights.com/news About Us: Coherent
Market Insights leads into data and analytics, audience measurement, consumer
behaviors, and market trend analysis. From shorter dispatch to in-depth insights,
CMI has exceled in offering research, analytics, and consumer-focused shifts
for nearly a decade. With cutting-edge syndicated tools and custom-made
research services, we empower businesses to move in the direction of growth. We
are multifunctional in our work scope and have 450+ seasoned consultants,
analysts, and researchers across 26+ industries spread out in 32+ countries. Contact
Us: Mr. Shah Coherent
Market Insights Pvt. Ltd, U.S.: +
12524771362 U.K.:
+442039578553 AUS: +61-8-7924-7805 INDIA:
+91-848-285-0837 ✉ Email: sales@coherentmarketinsights.com

