Montreal, Quebec--(Newsfile Corp. - November 4, 2025) - Defence Therapeutics Inc. (CSE: DTC) (FSE: DTC) (OTCQB: DTCFF) ("Defence" or the "Company"), a leading biotechnology company specialized in drug delivery technologies, is pleased to announce and to present today at the World ADC Conference in San Diego, USA, highly encouraging results from its latest preclinical in vivo study evaluating Accum®-Kadcyla, a novel version of Genentech/Roche's marketed ADC Kadcyla® (ado-trastuzumab emtansine), in mouse models of HER2-positive breast cancer.
Study Results: 20-Fold Increased Potency at Equivalent Dose
In the comparative in vivo study, Accum®-Kadcyla demonstrated a ~20-fold higher anti-tumor efficacy than Kadcyla® alone when administered at the same dose (0.5 mg/kg). Tumor growth was significantly halted in the Accum®-Kadcyla-treated group, resulting in a durable and near-complete response in most mice while Kadcyla® at the same dose (0.5 mg/kg) had no effect on tumor growth. Importantly, 100% of the animals survived throughout the duration of the study with no signs of toxicity, underscoring the excellent tolerability of the treatment (Figure below).
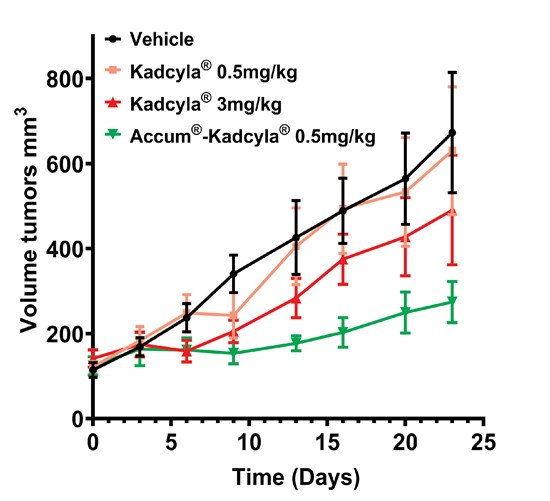
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/8000/272951_a8cb2e06a46e8ae0_002full.jpg
Implications for Patients and the Industry
These results confirm that Defence's Accum® platform can dramatically enhance the intracellular delivery and potency of ADCs by overcoming endosomal entrapment-a known bottleneck in ADC performance. By increasing the therapeutic payload's reach inside cancer cells, Accum® enables a more efficient drug release and tumor killing, even at lower doses.
This finding is particularly meaningful for patients: the ability to achieve the same or better efficacy at reduced doses translates into a potential reduction in toxicity and side effects, addressing one of the main limitations of current ADC therapies. Practically, it could potentially bring this current second line of treatment to a first line of treatment for the benefit of the patients.
Dr. Maxime Parisotto, Chief Scientific Officer of Defence Therapeutics, commented:
"These results further validate the power of Accum® as a transformative technology for ADCs. By amplifying the potency of a clinically proven ADC like Kadcyla® by 20 times at the same dose, we demonstrate that Accum® can unlock a new generation of safer and more effective targeted therapies for cancer patients."
Next Steps and Commercial Outlook
Following these promising results, Defence Therapeutics plans to expand its Accum®-ADC program to additional HER2-positive and HER2-low tumor models and to advance discussions with potential pharmaceutical partners.
About Defence:
Defence Therapeutics is a publicly-traded biotechnology company developing and engineering the next generation of ADC products using its proprietary platform. The core of Defence Therapeutics platform is the ACCUM® technology, which enables precision delivery of ADCs in their intact form to target cells. As a result, increased efficacy and potency can be reached against cancer.
For further information:
Sebastien Plouffe, President, CEO and Director
P: (514) 947-2272
Splouffe@defencetherapeutics.com
www.defencetherapeutics.com
Cautionary Statement Regarding "Forward-Looking" Information
This release includes certain statements that may be deemed "forward-looking statements". All statements in this release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects", "potential" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should" occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include regulatory actions, market prices, and continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company's management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management's beliefs, estimates or opinions, or other factors, should change.
Neither the CSE nor its market regulator, as that term is defined in the policies of the CSE, accepts responsibility for the adequacy or accuracy of this release.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/272951
