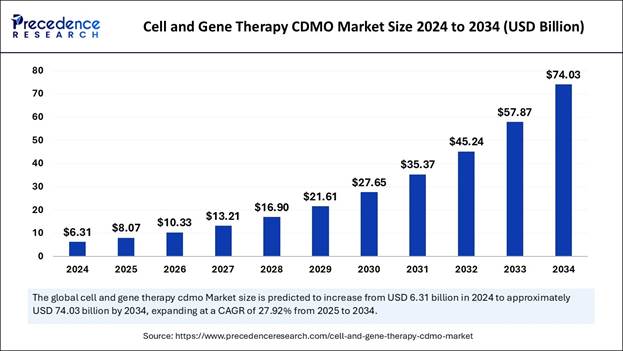
According to Precedence Research, the global cell and gene therapy CDMO market size is expected to be worth over USD 74.03 billion by 2034, increasing from USD 8.07 billion in 2025. The market is expected to expand at a healthy CAGR of 27.92% from 2025 to 2034.
The growing demand for specialized expertise, facilities,
and knowledge in the manufacturing and development of cell and gene therapies
is a major factor driving market growth.
The Complete Study is Now Available for Immediate Access | Download
the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/5567 Key Highlights of Cell and Gene Therapy CDMO Market
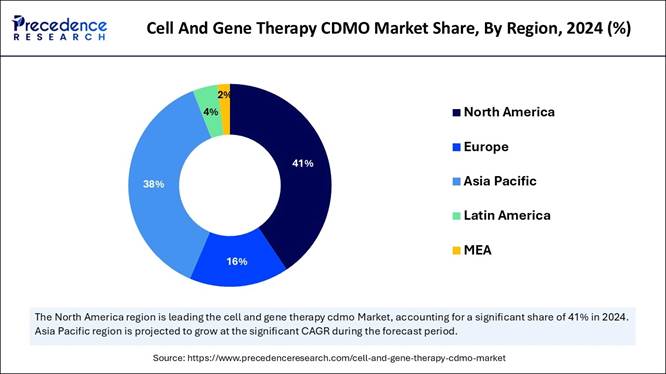
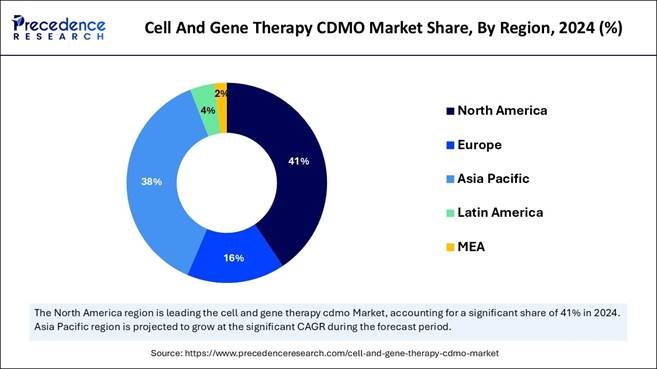
🔹 By region, North America dominated the cell and gene therapy CDMO
market in 2024, whereas the Asia Pacific is expected to grow in the foreseeable
period.
🔹 By phase,
the pre-clinical segment led the cell and gene therapy CDMO market in 2024,
whereas the clinical segment is expected to grow in the foreseeable period.
🔹 By product
type, the cell therapy segment led the cell and gene therapy CDMO market in
2024, whereas the gene-modified cell therapy
segment is expected to grow in the foreseen period.
🔹 By
indication, the oncology segment led the cell and gene therapy CDMO market in
2024, while the rare diseases segment is expected to grow over the forecast
period.
Advanced Technology Is Helpful for the Growth of the Cell
and Gene Therapy CDMO Industry
The cell and gene therapy CDMO market is growing mainly
due to the rising prevalence of infectious, chronic, and rare diseases
globally. Such diseases lead to a higher demand for precision medicine and treatment to achieve effective results and satisfied
patient outcomes.
Advanced technologies and an increasing number of clinical trials for the development of cell and gene therapies are major
drivers of market growth. CDMO provides expertise in specialized facilities for
the manufacturing of therapeutic products, including process development,
clinical trial products, and scaling up to commercial manufacturing. A
supportive regulatory framework and higher investment also help drive growth in
the cell and gene therapy CDMO market.
➡️ Become a valued research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting
New Trends of Cell and Gene Therapy CDMO Market
🔹Use of AI to
improve accuracy, scalability, and efficiency for manufacturing is one of the
major factors for the growth of the market.
🔹 Growing
regulatory frameworks, leading to higher demand for CDMOs to scale up
production from small clinical batches to large-scale commercial supplies, also
drive market growth.
🔹Emerging non-viral vectors, due to their low cost and
simpler production, also help to propel the growth of the market.
Role of AI in the Cell and Gene Therapy CDMO Market
Artificial intelligence (AI) is becoming a transformative force in the cell and gene therapy CDMO sector by streamlining
and optimizing complex biologics manufacturing and development processes. AI-powered tools
help in cell selection and quality control, enabling detection of potential
contamination, genetic inconsistencies, or deviations in cell behavior early in
the process, which reduces batch failures and improves overall yield and
safety.
Moreover, AI assists in viral vector design and
optimization, predicting gene expression outcomes and reducing time-consuming
trial-and-error during vector construct design. On the manufacturing side, AI
enables real-time process monitoring, predictive analytics,
and digital-twin
simulations, allowing CDMOs to scale production, manage supply chains more
efficiently, and uphold rigorous quality standards, thus accelerating
time-to-market for advanced therapies.
Unlock detailed insights on AI’s impact in the cell and
gene therapy CDMO market 👉 https://www.precedenceresearch.com/ai-precedence Cell and Gene Therapy CDMO Market Dynamics
Drivers
What Are the Growth Drivers of Cell and Gene Therapy CDMO
Market?
Factors such as the growing prevalence of chronic and
infectious diseases, the high demand for personalized medicine, and the increasing demand for targeted drug therapies
are major drivers of market growth. Living cells and genetic modifications in
rapidly expanding healthcare are also a vital factor in the market’s growth.
The market also helps drug developers to successfully prepare for the
successful execution of clinical trials for new and innovative therapies.
Hence, these are some of the major factors driving the market's growth.
How Do Manufacturing Complexities Restrain the Growth of
the Cell and Gene Therapy CDMO Market?
The inherent nature of therapies involving live cells,
viral vectors, and individual treatment plans, along with the complexities of
such therapies, is a major restraint on the market's growth. Hence, to manage
such issues, it is essential to maintain quality control, specialized expertise,
and adequate equipment. Increased production costs for approved gene therapies
are another restraint on the market's growth.
📥 Dive into the Complete Report ➡️ https://www.precedenceresearch.com/cell-and-gene-therapy-cdmo-market Opportunity
Specialized Facilities and Technologies Helpful for the
Growth of the Market
Higher research and investment in the development of
specialized facilities and technologies for successful, innovative cell and
gene therapies are major opportunities for market growth.
Partnering with a CDMO helps a biotechnological company gain expertise in
specific fields, further fueling market growth. It also helps manage drug
development and personalized medication and treatment, further fueling market
growth. Hence, such factors present a major opportunity for market growth.
Cell and Gene Therapy CDMO Market Report Coverage
Report Coverage Details Market Size in 2025 USD 8.07 Billion Market Size in 2026 USD 10.33 Billion Market Size by 2034 USD 74.03 Billion Market Growth Rate from 2025 to 2034 CAGR of 27.92% Dominating Region North America (67% market share in 2024) Fastest Growing Region Asia Pacific (CAGR of 29.03%) Base Year 2024 Forecast Period 2025 to 2034 Segments Covered Phase (Pre-clinical, Clinical), Product Type
(Cell Therapy, Gene-Modified Cell Therapy), Indication (Oncology, Rare
Diseases), Regions Key Segment Insights Pre-clinical phase dominated (67% share in
2024); Cell therapy led product type (42% share in 2024); Oncology led
indication (50% share in 2024); Rare diseases segment projected to grow
rapidly AI Integration Artificial intelligence enhances cell
selection, treatment prediction, large dataset analysis, and overall
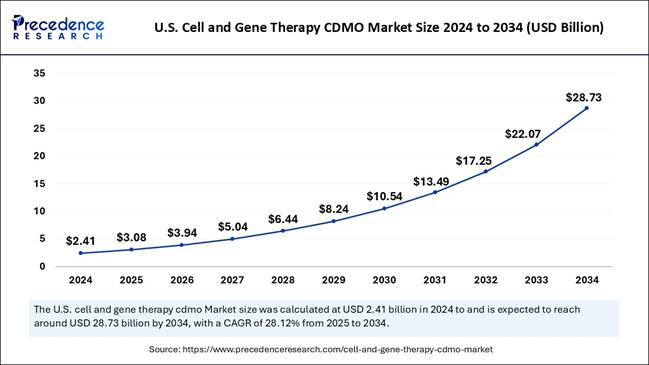
manufacturing efficiency U.S. Market Size USD 2.41 Billion in 2024; projected USD
28.73 Billion by 2034 (CAGR 28.12%) Growth Drivers Rising clinical trials, growing demand for
advanced therapies, AI-driven manufacturing, regulatory support, and
infrastructure in North America
For inquiries regarding discounts, bulk purchases, or
customization requests, please contact us at sales@precedenceresearch.com
Growing Cell and Gene Therapy Market Drives
Demand for CDMOs
The rapid expansion of the Cell and Gene Therapy Market is
fueling significant growth in the Cell and Gene Therapy CDMO (Contract
Development and Manufacturing Organization) Market.
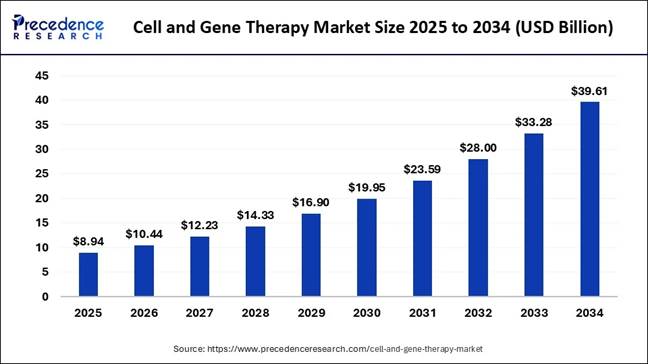
According to Precedence Research, the
global cell
and gene therapy market size is evaluated at
USD 8.94 billion in 2025 and is predicted to increase from USD 10.94 billion in
2026 to nearly USD 39.61 billion by 2034. The market is expected t grow at a
healthy CAGR of 17.98% from 2025 to 2034. As the demand for advanced therapies such
as CAR-T, gene-modified cells, and regenerative treatments rises, biopharmaceutical
companies increasingly rely on CDMOs for
specialized development and large-scale manufacturing.
Key Drivers Linking Both Markets:
🔹Rising Clinical Trials: Increasing number of cell and gene therapy
clinical trials worldwide has created a strong need for outsourced development
and manufacturing expertise.
🔹Complex Manufacturing Needs: Advanced therapies require sophisticated
processes for cell culture, viral vector production, and quality control,
making CDMOs essential partners.
🔹Cost and Time Efficiency: Outsourcing to CDMOs helps companies reduce
capital expenditure, accelerate time-to-market, and ensure regulatory
compliance.
🔹Technological Integration: Innovations in AI, automation, and gene-editing
tools adopted by CDMOs improve manufacturing precision and scalability,
directly benefiting therapy developers.
🔹Global Reach: Growth in regions like North America and Asia Pacific in
the therapy market is mirrored by expansion in CDMO capabilities to serve
regional demands efficiently.
As the Cell and Gene Therapy Market expands, CDMOs
are positioned as crucial enablers, bridging the gap between research
innovation and commercial-scale production, ensuring that life-saving therapies
reach patients faster and more reliably.
✚ Related Topics You May Find
Useful: ➡️ Cell and Gene Therapy
Manufacturing Market: Explore how advanced manufacturing technologies are enabling
large-scale production of innovative therapies. ➡️ Cell and Gene Therapy
Quality Control and Analytics Market: Discover the critical role of analytics and quality control in
ensuring safe and effective therapies. ➡️ Cell and Gene Therapy
Infrastructure and Delivery Models Market: Analyze emerging delivery models and
infrastructure solutions supporting therapy scalability. ➡️ Cell and Gene Therapy
Bioanalytical Testing Services Market: Understand how specialized testing services accelerate product
development and regulatory approval. ➡️ Cell Therapy Market: Track growth trends, key innovations,
and therapeutic applications driving the cell therapy sector. ➡️ Cell and Gene Supply Chain
Services Market: Explore how supply chain solutions optimize delivery and maintain
quality of advanced therapies. ➡️ Oncology CDMO Market: See how contract development and
manufacturing organizations are supporting the booming oncology therapy
pipeline. ➡️ Cell and Gene Therapy
Clinical Trials Market: Gain insights into trends and innovations shaping global clinical
trial activities. ➡️ Cell and Gene Therapy
Patient Access and Reimbursement Market: Understand how access strategies and
reimbursement policies are expanding patient reach and adoption. Cell and Gene Therapy Market Leading Companies ➢ Alnylam
Pharmaceuticals Inc. ➢ Biogen
Inc. ➢ CORESTEM
Inc. ➢ Dendreon
Pharmaceuticals LLC. ➢ Helixmith
Co. Ltd. ➢ JCR
Pharmaceuticals Co. Ltd. ➢ Kolon
TissueGene Inc. ➢ Novartis
AG ➢ Pfizer
Inc.
Note: This report is readily available for immediate delivery. We
can review it with you in a meeting to ensure data
reliability and quality for decision-making.
📥 Download Sample Pages for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/2445 Cell and Gene Therapy CDMO Market Regional Analysis
What is the U.S. Cell and Gene Therapy CDMO
Market Size?
The U.S. cell and gene therapy CDMO market
size is valued at USD 3.08 billion in 2025 and is expected to cross over USD
28.73 billion by 2034, growing at a strong CAGR of 28.12% from 2025 to 2034.
North America Led the Cell and Gene Therapy CDMO Market in
2024
North America led the cell and gene therapy CDMO market
in 2024 due to factors such as developed healthcare infrastructure, advanced
and technologically advanced facilities, expanding geographic research, a
supportive regulatory landscape, and high investment in research and
development.
Advanced technologies that are helpful for the development of new cell and gene
therapies are another major factor driving the market's growth. The US plays a
major role in the region's market growth due to improved healthcare facilities
and higher demand for precision medicine and treatments to achieve effective
results. The Full Study is Readily Available |
Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/5567
Asia Pacific Is Observed to Be the Fastest-Growing Region
in the Forecast Period
Asia Pacific is the fastest-growing region due to rising
clinical trials for cell and gene therapies, high demand for reliable, advanced
treatments, and a supportive regulatory framework, which are expected to drive
market growth in the foreseeable period.
Europe Is Observed to Have Notable Growth in the
Foreseeable Period
Europe is expected to experience notable growth in the
forecast period due to factors such as a robust biotechnology sector, an
increasing prevalence of genetic disorders and infectious diseases, and an
established healthcare infrastructure.
Cell and Gene Therapy CDMO Market Segmental Insights
By Phase Insights
The pre-clinical segment led the cell and gene therapy
CDMO market in 2024, driven by large and expanding pipelines of new and
innovative therapies, increased investment in research and development, and
high adoption of outsourcing for early-stage development services such as
vector design and small-scale manufacturing. The segment also observes growth,
providing various other services such as vector design, process development,
and small-scale manufacturing. Hence, these factors collectively help enhance
market growth.
The clinical segment is expected to grow over the
forecast period due to increasing clinical trials, the growing complexities of
such therapies, and the high demand for specialized manufacturing capabilities
to target rare diseases at their early stages. Complex cell and gene therapies
also drive higher demand for CDMO expertise in areas such as manufacturing,
process development, and quality control throughout the clinical process.
Hence, the segment isexpectedd to growovern theforecastn period.
By Product Type Insights
The cell therapy segment led the cell and gene therapy
CDMO market in 2024, driven by high demand in areas such as regenerative
medicine and personalized medicine. Many small and mid-sized biotech firms lack
the expertise needed for complex cell therapy manufacturing, leading to higher
demand for CDMOs and further fueling the market's growth. Hence, all such
factors contribute to the segment's growth.
The gene-modified cell therapy segment is expected to
grow over the forecast period due to the success of therapies such as CAR-T and
TCR-T, increased investment in personalized medicines and treatments, and
advancements in gene-editing technologies such as CRISPR. Expertise in the
segment for complex gene modification is another major factor driving market
growth in the foreseeable period.
By Indication Insights
The oncology segment led the cell and gene therapy CDMO
market in 2024, driven by the higher prevalence of chronic and infectious
diseases, such as cancer. Such factors further increase the demand for
personalized drugs and treatments, thereby fueling market growth. Higher demand
for cancer-targeted therapies, such as CAR-T, for effective treatment outcomes
is another major factor driving the market’s growth. Innovative and
technologically advanced cell and gene therapies are another major factor for
the market’s growth.
The rare diseases segment is expected to grow over the
forecast period due to the rising prevalence of infectious and chronic diseases,
driving high demand for personalized medications and treatments, thereby
supporting market growth. The ability of cell and gene therapies to address
genetic disorders is another major factor in the development of effective drugs
and treatments, helping the market’s growth in the foreseeable period. Factors
such as higher research and development spending, greater investment, and
orphan drug designation also help fuel the market’s growth over the forecast
period.
Recent Developments in Cell and Gene Therapy CDMO Market
🔸 In October
2025, Mytos launched its automated contract development and manufacturing
organization (CDMO), leveraging properties to overcome regenerative medicine’s
restrictions and enable scalable, affordable manufacturing of stem cell-derived
therapies. (Source- https://www.genengnews.com)
🔸In May 2025, Astraveus announced its plans to evaluate
its novel platform for CAR-T manufacturing, while a new global CDMO launched to
address development and manufacturing in the cell and gene industry. (Source-https://www.regmednet.com)
Top Companies of Cell and Gene Therapy CDMO Market
➢ Catalent ➢ Charles River Lobaoraties ➢ Curia ➢ Emergent BioSolutions ➢ Eurofins ➢ FUJIFILM Diosynth
Biotechnologies ➢ Genscript ➢ Lonza ➢ Pfizer CentreOne ➢ Recipharm ➢ Syngene ➢ Thermo Fisher Scientific ➢ Wacker ➢WuXi Biologics
Segments Covered in the Report
By Type of Therapy:
🔹 Gene
Therapy → Viral Vector-based
Gene Therapy → Non-viral Gene Therapy → Gene Editing Therapies 🔹 Cell
Therapy → Autologous Cell
Therapy → Allogeneic Cell
Therapy 🔹 Stem Cell Therapy 🔹 CAR-T
Therapy 🔹 Other Cell
Therapies
By Type of Manufacturing Service
🔹 Development
Services → Cell Line Development → Process Development → Analytical Testing → Regulatory Support 🔹 Manufacturing
Services
→ Clinical Manufacturing → Commercial
Manufacturing → Fill & Finish 🔹 Other
Services
→ Supply Chain
Management → Packaging and Labeling
By Cell and Gene Therapy Modality
🔹 Viral
Vectors
→ Adeno-associated Virus
(AAV) → Lentivirus → Adenovirus → Retrovirus → Other Viral Vectors 🔹 Non-Viral
Vectors
→ Plasmid DNA → mRNA-based Therapies 🔹 CRISPR/Cas9
and Other Gene Editing Tools
By Therapeutic Area
🔹 Oncology → CAR-T Therapies for
Cancer → Cancer Vaccines 🔹 Neurology → Gene Therapies for Neurological
Disorders → Gene Editing for
Neurological Conditions 🔹 Cardiovascular → Gene Therapy for Heart Diseases 🔹 Metabolic
Disorders → Gene Therapy for Rare Diseases 🔹 Other
Therapeutic Areas
→ Musculoskeletal → Ophthalmology → Infectious Diseases
By End-User
🔹 Biopharmaceutical
Companies →
Large pharma companies outsourcing
manufacturing → Small and medium-sized
biotech companies 🔹 Academic
and Research Institutions 🔹 Contract
Development & Manufacturing Organizations (CDMOs)
By Region
🔹 North America 🔹 Europe 🔹 Asia Pacific 🔹Latin America 🔹Middle East & Africa (MEA) Thanks for
reading you can also get individual chapter-wise sections or region-wise report
versions such as North America, Europe, or Asia Pacific. Don’t Miss
Out! | Instant Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/5567 You can place an
order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344 Stay Ahead with
Precedence Research Subscriptions Unlock exclusive access to powerful market
intelligence, real-time data, and forward-looking insights, tailored to your
business. From trend tracking to competitive analysis, our subscription plans
keep you informed, agile, and ahead of the curve. Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription About Us Precedence Research is a global market
intelligence and consulting powerhouse, dedicated to unlocking deep strategic
insights that drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we specialize in decoding the complexities of cell and gene therapy, drug development, and oncology
markets, helping our clients stay ahead in some
of the most cutting-edge and high-stakes domains in healthcare. Our expertise
spans across the biotech and pharmaceutical ecosystem, serving innovators,
investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care,
precision therapeutics, and beyond. Web: https://www.precedenceresearch.com ✚ Explore More Market Intelligence from Precedence Research: ➡️ Generative AI in Life Sciences: Explore how AI innovations are revolutionizing drug discovery,
research efficiency, and precision medicine. ➡️ Biopharmaceuticals Growth: Understand the accelerating expansion of biologics, therapeutic
proteins, and cutting-edge pharma pipelines. ➡️ Digital Therapeutics: Discover how technology-driven treatments are reshaping patient
care and improving clinical outcomes. ➡️ Life Sciences Growth: Gain insights into emerging opportunities, market expansion, and
innovation trends in the life sciences sector. ➡️ Viral Vector & Gene Therapy Manufacturing: Analyze the production advancements powering next-generation gene
therapies and precision medicine. ➡️ Wellness Transformation: See how consumer wellness trends are shaping supplements,
functional foods, and lifestyle-driven markets. ➡️ Generative AI in Healthcare: Unlocking Novel
Innovations in Medical and Patient Care:
Explore AI applications enhancing diagnostics, treatment personalization, and
patient engagement. Our Trusted Data Partners: Towards Healthcare | Nova
One Advisor | Onco Quant | Statifacts Get Recent News 👉 https://www.precedenceresearch.com/news For Latest Update Follow Us: LinkedIn | Medium | Facebook | Twitter
The cell and gene therapy CDMO market also observes growth in the region over
the expected timeframe, driven by China's adoption of advanced technology for
cell and gene therapy development. Government and regulatory support for trials
in the region is another major factor for the market’s growth.
High investments by major companies and the government in the development of
cell and gene therapies in the region also help fuel the market's growth.
Contributions from countries such as Germany and the UK, in the form of
advanced healthcare infrastructure and government support, also help fuel the
market's growth.