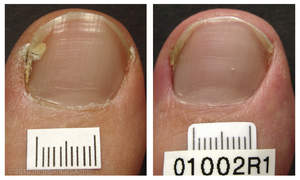
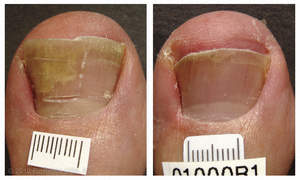
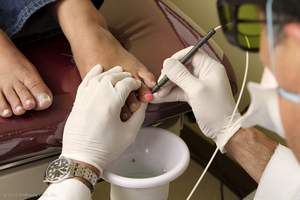
CHICO, CA--(Marketwire - October 20, 2010) - PinPointe USA, Inc., a leader in podiatric light-based therapy, announced today that the PinPointe™ FootLaser™ received clearance from the U.S. Food and Drug Administration (FDA) for the treatment of nail fungus (onychomycosis). During the procedure, which is administered by a podiatrist, a specially designed laser beam is directed across the nail. The laser penetrates the nail bed, targeting the fungi responsible for the infection, while leaving the nail and surrounding healthy tissue intact.
Fungal nail infection is estimated to affect more than 10 percent of the U.S. population -- or 35 million Americans. The condition is caused by fungus under the nail. As they grow, fungi feed on keratin, the tough protein that makes up the hard surface of the nails. The nail becomes darker in color and debris may accumulate under the nail. And the infection may spread to other toenails, the skin or the fingernails. Nail infection can cause nails to be discolored, thickened, brittle, and 'crumbly' and in some people it can interfere with wearing shoes and/or cause pain when walking.
"Toenail fungus is an incredibly embarrassing, chronic condition affecting millions of people worldwide that genuinely impacts a person's quality of life. For some with diabetes or immune disorders, nail fungus can lead to serious health problems," said Dr. Adam Landsman, Assistant Professor of Surgery at Harvard Medical School and Chief of the Division of Podiatric Surgery at Cambridge Hospital in Massachusetts. "With the clearance of the PinPointe FootLaser, patients finally have a pain-free treatment option that is more successful than topically applied antifungal drugs, safer than oral medication, and less painful than surgical removal of the nail."
The treatment of fungal nail infection is difficult because the infection is under and inside of the nail. This makes it hard for any treatment to reach and destroy the infection. Some people are treated with oral medications, which can be associated with side effects and serious drug interactions, or medicated nail polish. Another option is to surgically remove the nail. Many people also try bleach, vinegar, mouthwash or household cleaners -- home remedies that ultimately do not resolve the problem.
"PinPointe submitted clinical evidence to FDA demonstrating that after a single treatment, between 68 percent and 81 percent of patients experienced increased clear nail at six and 12 months and 81 percent of all patients had sustained improvement at 12 months. Now, a single 30-minute treatment can be done comfortably in the privacy of a doctor's office without the need for anesthesia," says John Strisower, founder and Chief Executive Officer of PinPointe. "Our goal is continued market leadership of podiatric light-based therapeutic technology."
The PinPointe FootLaser is also sold in the European Union under a CE Mark for the treatment of nail fungus, and is TGA-approved and available in Australia and New Zealand.
About PinPointe USA, Inc.
PinPointe USA, Inc., located in Chico, California, develops and markets innovative laser-based solutions for podiatrists and patients worldwide. The PinPointe FootLaser for treating nail fungus and other conditions is available in nearly every major metropolitan area in the United States. All PinPointe products provide superior clinical performance which is the result of years of research, sound science and clinical studies.
For patients wanting additional information or to find a doctor in their area offering the PinPointe FootLaser procedure, call (877) Toe-Nail or (877) 763-6245 or visit www.PinPointeFootLaser.com/find. For podiatrists interested in becoming a PinPointe provider, call (530) 809-3809 or visit www.PinPointeUSA.com.
WCG Contacts
Karen Halsey
Account Director
WCG
415-946-1077 (office)
415-272-5107 (mobile)
Email Contact
Julie Dye
Account Director
WCG
512-961-7524 (office)
512-563-7261 (mobile)
Email Contact