OliX Pharmaceuticals, Inc. (KOSDAQ: 226950), a leading developer of RNAi therapeutics, today announced positive headline results of a phase 2a trial in the treatment of hypertrophic scars which met the primary observer-based endpoint at week 24 as evaluated by the Patient and Observer Scar Assessment Scale (POSAS).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230428005123/en/
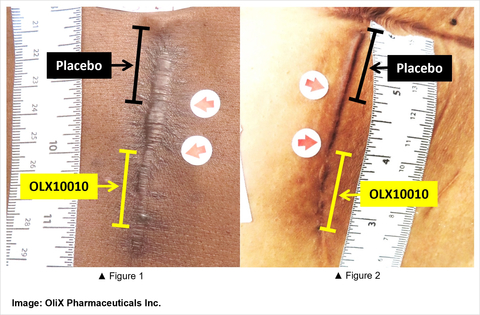
OLX10010 for Hypertrophic Scar Treatment (Image: OliX Pharmaceuticals Inc.)
The data shows that 24 weeks after scar excision surgery relative to baseline, the biweekly intradermal administration of OLX10010, but not placebo, leads to significant improvement of hypertrophic scar appearance (see Figure 1 + 2) on both, the POSAS observer global score (p=.0017, 2-sided) and the POSAS observer total score (p=.029, 2-sided). There was no clear evidence for dose-dependent (2mg and 5mg) effects. Follow-up of participating patients up to 48 weeks post-surgery is ongoing.
The trial confirms the safety and tolerability of OLX10010 at both dose levels, with no safety issues identified. Also, no negative effects on wound-healing after surgery were observed. The absence of any safety concerns stimulates development of OLX10010 as monotherapy or in combination with other treatments for hypertrophic scars, a condition for which currently available treatments are deemed insufficient.
Dr Waibel, University of Miami Miller School of Medicine and Subsection Chief of Dermatology, Baptist Hospital of Miami, and Miami Dermatology & Laser Institute, who participated in this trial stated: “The treatment of hypertrophic scars remains elusive to date with no targeted treatments available for this large patient population. Connective tissue growth factor (CTGF) plays an important role in fibrosis. CTGF inhibitors, like OLX10010, have inherent advantages with providing an evidence-based treatment approach and practical advantages of allowing new mechanisms of action and less frequent administration. The safety data observed in this trial are particularly encouraging positioning OLX10010 as potential first-in-line treatment for this indication.”
Dong Ki Lee, Ph.D., Founder and Chief Executive Officer of OliX Pharmaceuticals, said: “Hypertrophic scarring is a significant concern for patients and a challenging problem for clinicians. With at present no treatment modalities allowing sufficient treatment of hypertrophic scars, this condition presents a major unmet medical need with a significant compound annual growth rate. We are excited about the initial results from this trial, supporting the development of OLX10010 as backbone treatment for patients with hypertrophic scars, when used alone and in combination with standard of care and future novel treatments.”
About OLX10010
OLX10010 is an asymmetric siRNA duplex with a cell-penetrating moiety attached to the 3’ of sense strand (cell-penetrating asymmetric siRNA, i.e. cp-asiRNA), targeting human connective tissue growth factor (CTGF), which is a key factor in the development of hypertrophic scars. OLX10010 can be delivered into cells without any delivery systems, and interferes with the expression of CTGF with complementary nucleotide sequences by degrading mRNA after transcription. Drug candidates targeting CTGF support CTGF as a promising therapeutic target for the treatment of fibrotic diseases, i.e., hypertrophic scars. The use of OLX10010 is currently under clinical investigation and the safety and efficacy have not yet been fully evaluated by the US Food and Drug Administration (FDA) or any other regulatory agency.
About OliX Pharmaceuticals
OliX Pharmaceuticals is a clinical-stage pharmaceutical company developing therapeutics against a variety of disorders by down-regulating the expression of disease-causing genes based on its own proprietary RNAi technology. The Company’s core RNAi platform, asymmetric siRNA (asiRNA), is a unique gene silencing technology based on RNA interference (RNAi), which is considered the most efficient gene silencing technology. Utilizing this proprietary asiRNA technology, OliX has developed cell penetrating asiRNA (cp-asiRNA), a therapeutic RNAi platform to effectively target diseases including hypertrophic scarring, dry and wet age-related macular degeneration (AMD), and subretinal fibrosis. OliX has also developed another therapeutic RNAi platform, GalNAc-asiRNA, to target a variety of liver diseases.
Learn more: www.olixpharma.com/eng
View source version on businesswire.com: https://www.businesswire.com/news/home/20230428005123/en/
Source: OliX Pharmaceuticals







