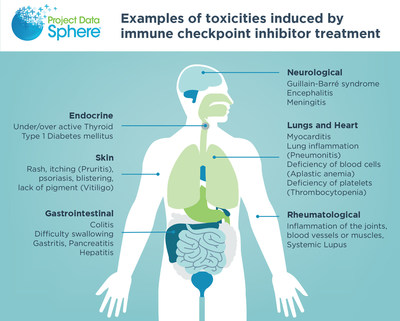
A paper outlining consensus disease definitions for neurological toxicities in cancer patients undergoing immune checkpoint inhibitor treatment was published this week in the Journal for ImmunoTherapy of Cancer (JITC) by a team of physicians at 16 institutions (https://pubmed.ncbi.nlm.nih.gov/34281989/).
|
CARY, N.C., July 28, 2021 /PRNewswire/ -- A paper outlining consensus disease definitions for neurological toxicities in cancer patients undergoing immune checkpoint inhibitor treatment was published this week in the Journal for ImmunoTherapy of Cancer (JITC) by a team of physicians at 16 institutions (https://pubmed.ncbi.nlm.nih.gov/34281989/). Neurologists and oncologists at Massachusetts General Hospital with support from scientists at Project Data Sphere® led the team to deliver the first consensus-based definitions of disease and severity criteria for any immune-related toxicity. These neurologic disease definitions function as proof of concept for toxicities affecting other organ systems. Neurologic immune related adverse events (irAEs) develop in an estimated 1-12% of cancer patients treated with immune checkpoint inhibitors (ICIs). Although neurological immune-related adverse events (irAE-Ns) are considered among the uncommon toxicities, they have a higher fatality rate compared with other irAEs. Over 35% of all patients with cancer are currently eligible for treatment with an ICI, and that number is likely to grow with expanding FDA approved indications. The lack of applicable disease definitions and severity criteria has made it difficult to diagnose these toxicities, negatively affects patient care, and impedes progress in clinical and translational research. The consensus definitions and severity criteria will serve as the framework for a multicenter neurologic irAE registry housed at Mass General Hospital. Project Data Sphere is committed to data sharing and will continue to seek to bring stakeholders together and make de-identified data publicly accessible. The registry will provide an important dataset that, along with governmental, international, and company-based datasets, can be explored to identify ICI-related toxicities in groups of patients that are typically larger and more heterogeneous than those included in clinical trials. The disease definitions and severity criteria were drafted by a team of four neurologists at Massachusetts General Hospital and then revised by a panel of 27 neurologists, oncologists, neuro-oncologists, and irAE subspecialists located at academic medical centers across the United States. One oncologist was located in Europe. The revision process involved two rounds of anonymous voting and two meetings to discuss areas of disagreement. At the end of the process, all disease definitions and guidance statements reached consensus among the panelists. Events in this category include those that affect the peripheral nervous system – neuropathy, myasthenia gravis, myopathy – and those that affect the central nervous system – meningitis, encephalomyelitis, demyelinating disease, and vasculitis. Eight ICIs have been approved by the U.S. Food and Drug Administration to treat more than 70 cancer indications, and new ICIs are actively being studied in clinical trials. The development of severe irAEs can limit the ability of patients to complete ICI treatment and receive the full benefit from this therapy. Experts representing a wide variety of sectors, from academic medical centers to biotech and pharmaceutical companies to governmental regulatory agencies, have called for the urgent need to strengthen the collection and analysis of data, both from clinical trials and real-world settings, to improve the understanding and management of irAEs. This need was the topic of a 2019 symposium co-sponsored by the FDA and Project Data Sphere. A call to action was published concurrently in JITC with the neurologic irAE consensus definitions. (https://jitc.bmj.com/content/9/7/e002896) About Project Data Sphere PDS is an independent initiative of the CEO Roundtable on Cancer, is a 501(c)(3) nonprofit corporation founded by President George H.W. Bush in 2001 to develop and implement initiatives that reduce the risk of cancer, enable early diagnosis, facilitate access to the best available treatments, and hasten the discovery of novel and more effective anti-cancer therapies. In addition to operating an open-access data-sharing platform, PDS manages research programs that explore leading issues in oncology using machine learning tools and big data analytics. For more information, visit ProjectDataSphere.org.
SOURCE Project Data Sphere |