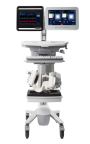
Neural Analytics, Inc. announced the first subjects enrolled with its next-generation robotic platform as part of the IRB approved CODEX study, a prospective, single arm, multi-center, safety and technical feasibility study utilizing an investigational device exemption version of the Lucid™ Robotic System, at the Ochsner Clinical Foundation in New Orleans, Louisiana.
LOS ANGELES--(BUSINESS WIRE)-- Neural Analytics, Inc., a medical robotics company developing and commercializing technologies to measure and track brain health, announced today the first subjects enrolled with its next-generation robotic platform as part of the IRB approved CODEX study, a prospective, single arm, multi-center, safety and technical feasibility study utilizing an investigational device exemption(IDE) version of the Lucid™ Robotic System (Lucid™ M1 Transcranial Doppler Ultrasound System® and NeuralBot™ System), at the Ochsner Clinical Foundation in New Orleans, Louisiana. The new IDE version of the technology is intended to improve performance and ease of use for the company’s robotic platform to further assist physicians in management of patients suspected of suffering stroke.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191031005319/en/
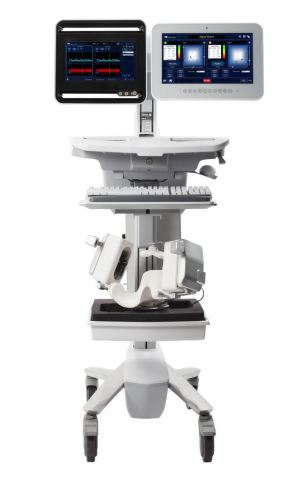
The Lucid Robotic System (Photo: Business Wire)
“We are honored to be the first in the world to evaluate the performance of Neural Analytics’ next-generation robotic technology. So far, we have been impressed with the improved simplicity, and ease of use of the new Lucid™ Robotic System,” said Ifeanyi Iwuchukwu, MD, assistant professor of Neurocritical Care and Stroke at The Ochsner Clinical Foundation. “Over the past nine months we have generated a tremendous amount of data to support the use of the Lucid Robotic System in the management of patients suffering stroke and we are excited to be building upon that with this next generation platform.”
Dr. Iwuchukwu and the Ochsner Clinical Foundation are the first to clinically evaluate the new version of the Lucid Robotic System. The first generation of the Lucid™ Robotic System was FDA cleared in 2018 (K180455), and Dr. Iwuchukwu and the Ochsner Clinical Foundation have been using an investigational version of the device within the CODEX Study System to gather and further explore the technical feasibility of the novel technology in a wide range of patients and neurological pathologies where the monitoring of cerebral blood flow velocities (CBFV) would be of benefit to stroke clinicians.
“We are grateful to be able to continue our partnership with Dr. Iwuchukwu and the Ochsner Clinical Foundation in the evaluation of our next-generation Lucid™ Robotic System,” said Robert Hamilton, Ph.D., Co-Founder and Chief Science Officer of Neural Analytics. “This milestone represents our continued commitment to providing the clinical community with critical tools to evaluate suspected stroke patients using our robotic, autonomous and non-invasive patient management platform.”
Each year, neurological diseases cost the U.S. healthcare system nearly $800 billion.2 Traumatic brain injury, migraine, Alzheimer’s and stroke account for more than $438 billion of that cost.1 Stroke affects more than 795,000 people in the U.S. - someone has a stroke every 40 seconds, and someone dies from stroke every 4 minutes.2 It is a time sensitive disease and requires intervention within 24 hours of onset of symptoms.3 Incorrect assessment of large vessel stroke leads to misdiagnosis and treatment delays, resulting in death or disability for stroke patients. Despite recent advances in life-saving treatments for acute ischemic stroke, less than five percent of stroke patients qualify for intervention because they do not present early enough.4 The American Heart Association/American Stroke Association Guidelines for Early Management of Acute Ischemic Stroke state that detection of large vessel occlusion by means of noninvasive intracranial vascular imaging greatly improves the ability to make appropriate clinical decisions.5
The Lucid Robotic System is a medical ultrasound device intended for use as an adjunct to standard clinical practices for measuring and displaying CBFV and the occurrence of transient emboli within the brain. The device is not intended to replace other means of evaluating vital patient physiological processes. Neural Analytics is actively enrolling patients into this research feasibility study and intends on activating up to 15 sites, both domestically and globally.
About Ochsner Clinical Foundation
Ochsner Health System is Louisiana’s largest non-profit, academic, healthcare system. Driven by a mission to Serve, Heal, Lead, Educate and Innovate, coordinated clinical and hospital patient care is provided across the region by Ochsner's 40 owned, managed and affiliated hospitals and specialty hospitals, and more than 100 health centers and urgent care centers. Ochsner is the only Louisiana hospital recognized by U.S. News & World Report as a “Best Hospital” across three specialty categories caring for patients from all 50 states and more than 70 countries worldwide each year. Ochsner employs nearly 25,000 employees and over 4,500 employed and affiliated physicians in over 90 medical specialties and subspecialties and conducts more than 700 clinical research studies. Ochsner Health System is proud to be a tobacco-free environment.
For more information, please visit www.ochsner.org and follow us on Twitter and Facebook.
About Neural Analytics, Inc.
Neural Analytics, Inc. is a medical robotics company developing and commercializing technologies to measure and track brain health. The company’s Lucid Robotic System (Lucid™ M1 Transcranial Doppler Ultrasound System® and NeuralBot™ System) is a robotically assisted ultrasound system for brain health assessment. It combines an all-in-one neurovascular ultrasound device, designed to non-invasively measure and display brain blood flow information under the guidance of a healthcare professional. The company’s technology integrates ultrasound, robotics and machine learning to empower neurologists with critical information about brain health to make clinical decisions and improve patient outcomes.
More information is available at http://www.neuralanalytics.com
_______________________
1 Clifton L. Gooch, Etienne Pracht, Amy R. Borenstein. The Burden of Neurological Disease in the United States: A Summary Report and Call to Action. Annals of Neurology, 2017; DOI: 10.1002/ana.24897
2 Center for Disease Control. Stroke Facts. https://www.cdc.gov/stroke/facts.htm. Accessed April 25, 2018
3 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018; January 24
4 Christopher R. Bernheisel, MD, Jeffrey D. Schlaudecker, MD, Katelyn Leopold, MD. Subacute Management of Ischemic Stroke. American Family Physician, 2011 Dec 15;84(12):1383-1388
5 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013; February 25
View source version on businesswire.com: https://www.businesswire.com/news/home/20191031005319/en/
Contacts
General Inquires
877-638-7251
Info@neuralanalytics.com
Investor Relations
Neural Analytics, Inc.
Artin Davidian, Director of Finance
IR@neuralanalytics.com
Source: Neural Analytics, Inc.
Smart Multimedia Gallery
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20191031005319/en