Continuous glucose monitoring demonstrates improved time-in-range, key metabolic datacompared to hybrid closed-loop systems [08-June-2019] MIAMI , June 8, 2019 /PRNewswire/ -- Continuous glucose monitoring (CGM) evaluations in islet transplant recipients who have been insulin independent for an average of 10 years show near-normal glycemic profiles and time-in-range m
Continuous glucose monitoring demonstrates improved time-in-range, key metabolic datacompared to hybrid closed-loop systems
MIAMI, June 8, 2019 /PRNewswire/ -- Continuous glucose monitoring (CGM) evaluations in islet transplant recipients who have been insulin independent for an average of 10 years show near-normal glycemic profiles and time-in-range metrics, according to data presented by the Diabetes Research Institute at the University of Miami Miller School of Medicine. The findings, which were accepted as a late-breaking poster at the American Diabetes Association (ADA) 79th Scientific Sessions, June 7 – 11, 2019 in San Francisco, CA, demonstrate that islet transplantation can be a successful long-term cell therapy for select patients with type 1 diabetes.
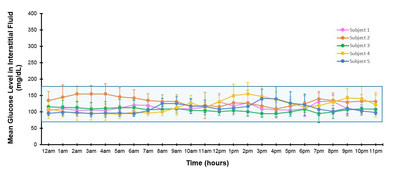
The DRI team evaluated five of its adult subjects who received intrahepatic (in the liver) islet transplants between 2002 – 2010 and have since remained insulin independent for seven to 16+ years. During their last study follow-up, the subjects completed a 7-day, non-blinded CGM to assess their glycemic profiles. Compared to current recommended CGM goals for adults with type 1 diabetes on a hybrid closed-loop system, all patients demonstrated improved CGM time-in-range, reduction in glucose variability, and prevention of hypoglycemia. A sampling of the results is as follows:
|
CGM Glucose Range |
CGM % Time-in-Range |
CGM % Time-in-Range in DRI islet |
|
70 - 180 |
≥70 |
96.4 |
|
<70 |
≤3 |
0.9 |
|
<54 |
≤1 |
0.1 |
In addition, time in the more stringent glucose range of 70-140 mg/dL was 83.1%, with a mean sensor glucose (SG) value of 116 mg/dL and an average HbA1c of 5.7%. The ADA's recommended HbA1c goal is <7% for adults with diabetes.
"Using continuous glucose monitoring, we now have the ability to accurately evaluate patients' glucose profiles and their variability. The CGM data we have obtained from our islet transplant patients clearly demonstrates that islet transplantation can result in glucose levels that are close to those in people who do not have type 1 diabetes, even 10 years or more after undergoing the cell-replacement procedure," said David Baidal, M.D., assistant professor of medicine and member of the DRI's Clinical Islet Transplant Program. One of the principal investigators of the study, Dr. Baidal is presenting the results at the ADA conference.
"Although not all subjects remain insulin independent, like the subjects described in this presentation, after an islet transplant a significant number of them continue with excellent graft function for over 10 years that allows them to have near-normal glucose metabolism in the absence of severe hypoglycemia on small doses of insulin," said Rodolfo Alejandro, M.D., director of the Clinical Cell Transplant Program and also a principal investigator of the study. Dr. Alejandro will be presenting these results at the upcoming 17th World Congress of the International Pancreas & Islet Transplant Association, July 2-5, 2019 in Lyon, France.
"This report confirms the superiority of transplantation of insulin-producing cells compared to insulin therapy, with glucose control results that were even better than the goals of CGM in hybrid closed-loop systems. Hopefully, this will be of assistance in bringing islet transplantation closer to FDA approval, allowing the treatment to be made available to U.S. patients, as has already been the case in several other countries, for many years," said Camillo Ricordi, M.D., Stacy Joy Goodman Professor of Surgery and director of the Diabetes Research Institute, who was recently named the world's leading expert in islet transplantation by Expertscape. Dr. Ricordi is well-known for inventing the machine (Ricordi Chamber) that made it possible to isolate large numbers of islet cells from the human pancreas and for performing the first series of successful clinical islet transplants that reversed diabetes after implantation of donor purified islets into the liver of recipients with diabetes.
In type 1 diabetes, the insulin-producing islets cells of the pancreas have been mistakenly destroyed by the immune system, requiring patients to manage their blood sugar levels through a daily regimen of insulin therapy. Islet transplantation has allowed some patients to live without the need for insulin injections after receiving a transplant of donor cells. Some patients who have received islet transplants have been insulin independent for more than a decade, as DRI researchers have published. Currently, islet transplantation remains an experimental procedure limited to a select group of adult patients with type 1 diabetes.
In 2016, the National Institutes of Health-sponsored Clinical Islet Transplantation Consortium reported results from its Food and Drug Administration (FDA)-authorized Phase 3 multi-center trial, of which the DRI was a part, indicating that islet transplantation was effective in preventing severe hypoglycemia (low blood sugar levels), a particularly feared complication in type 1 diabetes that can lead to seizures, loss of consciousness and even death. The study was a significant step toward making islet transplantation an approved treatment for people with type 1 diabetes and reimbursable through health insurance, as it is in several other countries around the world.
Funding has been provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) grant U01-DK-070460; Immune Tolerance Network grant NIS01; Diabetes Research Institute Foundation; State of Florida; University of Miami Clinic and Translational Science Institute grant 1UL1TR000460; and JDRF (4-2004-361)
About the Diabetes Research Institute
The Diabetes Research Institute at the University of Miami Miller School of Medicine leads the world in cure-focused research. As one of the largest and most comprehensive research centers dedicated to curing diabetes, the DRI is aggressively working to develop a biological cure by restoring natural insulin production and normalizing blood sugar levels without imposing other risks. Researchers have already shown that transplanted islet cells allow patients to live without the need for insulin therapy. Some study participants have maintained insulin independence for more than 10 years. The DRI is now building upon these promising outcomes through its BioHub strategy, a multidisciplinary, three-pronged approach for addressing the major challenges that stand in the way of a cure: eliminate the need for anti-rejection drugs, reset the immune system to block autoimmunity, and develop an unlimited supply of insulin-producing cells. For more information, please visit DiabetesResearch.org, call 800-321-3437, or Tweet @Diabetes_DRI.

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/islet-transplant-recipients-with-long-term-insulin-independence-show-near-normal-glucose-control-300864035.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/islet-transplant-recipients-with-long-term-insulin-independence-show-near-normal-glucose-control-300864035.html
SOURCE Diabetes Research Institute Foundation




