CARLSBAD, Calif., Jan. 7, 2016 /PRNewswire/ -- ImpediMed Limited (ASX: IPD), a global provider of medical technology to measure, monitor and manage fluid status in patients, has announced the U.S. commercial launch of its L-Dex® system to aid in the clinical assessment of lymphedema.

This follows a successful pilot program involving six U.S. cancer centers, a dedicated CPT® Category I code enabling physicians to seek reimbursement beginning in January 2015, and a revised, unencumbered Food and Drug Administration (FDA) clearance in May 2013.
L-Dex is the first product of its kind to utilize bioimpedance spectroscopy (BIS), a non-invasive system for accurately measuring tissue composition and fluid status, to identify lymphedema up to 10 months before there is evidence of limb swelling. Early detection and subsequent intervention may help prevent the progression of the disease and, in some instances, even reverse it.
"Fluid monitoring is a critical component in the continuum of care for cancer survivors," said Richard Carreon, Managing Director and CEO of ImpediMed. "L-Dex is an accurate measurement tool, which can help improve health outcomes for patients through earlier identification of lymphedema."
An estimated three to five million people in the U.S. live with lymphedema as the result of impaired lymphatic systems due to trauma, most commonly surgery or radiation therapy from cancer treatment. In July 2015, the National Comprehensive Cancer Network® (NCCN®) recognized lymphedema as a long-term chronic condition and outlined guidelines for screening the disease among its alliance of 26 leading cancer centers.
Gathering evidence on the benefits of BIS technology for early detection
Through electrodes placed on the arm and leg, L-Dex sends a small, imperceptible current using 256 frequency spectra through tissue measuring extracellular fluid. Using a scoring system, oncologists, breast cancer surgeons and other healthcare providers can determine in seconds the amount of resistance from one point to the other and identify the onset of extracellular fluid accumulation, which may assist in the identification of lymphedema at an earlier point in time.
An independent clinical trial conducted at Magee-Womens Hospital of UPMC between 2010 and 2013 found that BIS technology helped reduce the clinical lymphedema rate from 36.4 percent to 4.4 percent. A randomized controlled study using L-Dex is currently underway at six leading cancer centers, including the Vanderbilt-Ingram Cancer Center, University of Texas MD Anderson Cancer Center, Mayo Clinic, University of Kansas Cancer Center, VCU Massey Cancer Center and Macquarie University. Interim results of this study will be released in late 2016, increasing the clinical evidence that supports the vital role of BIS in early identification of lymphedema in patients being treated for breast cancer and other cancers.
ImpediMed raised over AU$40 million in funding from institutional and private investors, including FIL Investment (Fidelity), over the past two years to support the U.S. commercial launch of L-Dex. Through the acquisition of key assets and intellectual property from Carlsbad, California-based Intersection Medical, the company will also explore potential uses for BIS technology in the diagnosis and monitoring of chronic heart failure and other chronic conditions.
"The launch of L-Dex across the U.S. moves ImpediMed into the next stage of its clinical journey," said Carreon. "Our objective is to not only make L-Dex the standard of care in the clinical assessment of early stage lymphedema, but to explore its potential in other areas where a simple, sophisticated platform could accurately measure and monitor fluid levels leading to better patient outcomes."
For more information on the L-Dex system for early assessment of lymphedema, patients and physicians may visit www.impedimed.com or call toll-free 877-247-0111. Follow ImpediMed on LinkedIn, YouTube, Facebook and Twitter.
About ImpediMed
Founded and headquartered in Brisbane, Australia with U.S. offices in Carlsbad, California, ImpediMed is the world leader in the development and distribution of medical devices employing bioimpedance spectroscopy (BIS) technologies for use in the non-invasive clinical assessment and monitoring of fluid status in patients. ImpediMed has the first medical device with FDA clearance in the U.S. to aid healthcare professionals to clinically assess secondary unilateral lymphedema of the arm and leg in women and the leg in men. For additional information, visit www.impedimed.com.
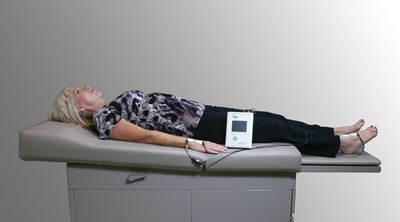
Photo - http://photos.prnewswire.com/prnh/20160107/320112
Photo - http://photos.prnewswire.com/prnh/20160107/320111
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/impedimed-announces-us-commercial-launch-of-l-dex-system-for-assessment-of-lymphedema-in-cancer-survivors-300201107.html
SOURCE ImpediMed





