Ocean Biomedical and Aesther Healthcare Acquisition Corp. announced the discovery of bispecific antibodies that target Chitinase 3-like-1 and immune checkpoint inhibitors, killing glioblastoma cells and melanoma cells, and blocking the metastasis of malignant melanoma cells to the lung by over 90%.
• Ocean Biomedical will be a wholly owned subsidiary of Aesther Healthcare Acquisition Corp. and will change its name to Ocean Biomedical, Inc., expected to be listed on NASDAQ under the symbol, “OCEA”.
Providence, RI and New York, NY, Sept. 06, 2022 (GLOBE NEWSWIRE) -- Ocean Biomedical and Aesther Healthcare Acquisition Corp.(“Aesther”) (NASDAQ: AEHA) announced today the discovery of bispecific antibodies that target Chitinase 3-like-1 and immune checkpoint inhibitors, killing glioblastoma cells and melanoma cells, and blocking the metastasis of malignant melanoma cells to the lung by over 90%. Glioblastoma multiforme (GBM) is a deadly type of brain tumor and 5-year survival is just 8% for those aged 45-54. About 25% of GBM patients are not actively treated due to rapid disease progression. Malignant melanoma, the most serious skin cancer, can metastasize to other organs. Once it has spread to other organs it is difficult to treat. Metastatic melanoma (Stage IV) has 22.5% five year survival. Non-small cell lung cancer (NSCLC) is a major unmet medical need that accounts for 85% of pulmonary malignancies and effects approximately 450,000 individuals. In greater than 50% of affected patients the tumors are diagnosed at advanced stages with metastatic spread that precludes curative surgical resection.
Background
Recent studies of NSCLC have highlighted genetic abnormalities that underlie these tumors. These genetic abnormalities generate abnormal proteins that have not been previously seen by the patient’s immune system which, in turn, activates antitumor immune responses that control tumor initiation and progression. Studies over recent years have demonstrated that tumor initiation and progression are often mediated by the ability of the tumors to produce immunosuppressive proteins and activate immunosuppressive pathways, called immune checkpoint inhibitors (ICPI), that allow tumor growth and progression by shutting off these critical anti-tumor immune responses. This includes the programed death (PD) pathway including PD-1, PD ligand 1 (PD-L1) and PD-L2 and the cytotoxic T-lymphocyte-associated protein 4 (CTLA4) pathway that includes CTLA4 and its binding partners B7.1/B7.2. Antibodies that target ICPI such as PD-1, PD-L1 and CTLA4 have been generated which have therapeutic efficacy in NSCLC and other tumors. Unfortunately, only a minority of patients respond to these therapies and the responses are often not durable.
Chitinase 3-like-1 (CHI3L1) is a member of the 18 glycosyl hydrolase gene family that is readily detected in the circulation of normal individuals and expressed at exaggerated levels in the circulation of individuals with diseases characterized by inflammation, tissue remodeling and or cancers.
Discoveries
Recent studies from our laboratory have demonstrated that CHI3L1 is a critical regulator of a number of key cancer-causing pathways. We have highlighted its ability to inhibit tumor cell death (apoptosis), its inhibition of the expression of the tumor suppressors P53 and PTEN and its stimulation of the B-RAF protooncogene. Most recently we have discovered that CHI3L1 is a “master regulator” of ICPI including key elements of the PD-1 and CTLA4 pathways. In accord with the importance of these pathways we have also generated antibodies: 1.) a monoclonal antibody against CHI3L1, and 2.) bispecific antibodies that simultaneously target CHI3L1 and PD-1 or CTLA4. The impressive ability of our bispecific antibodies to control primary and metastatic lung cancer in murine experimental modeling systems is discussed below.
We generated bispecific antibodies thatsimultaneously target CHI3L1 and PD-1 or CTLA4. We then compared their effects in experimental models in which T cells and tumor cells are cultured together (a co-culture system) and in a murine model of lung metastasis. In all cases we compared the effects of the bispecific antibody to control antibodies, and to individual monospecific antibodies against CHI3L1, PD-1 or CTLA4, alone or in combination and the results are shown in the figures below.
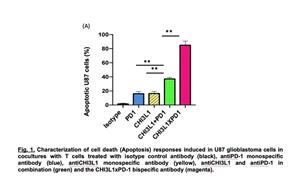
In the coculture system, critical immune regulating cells called T cells were placed in culture with cancer cells. The ability of the antibodies to induce T cell differentiation and kill (induce apoptosis) of the tumor cells were then evaluated. As can be seen in Figure 1 below, tumor cell death was not induced by isotype control antibodies, and modest degrees of tumor cell apoptosis were seen in cultures with monospecific antiCHI3L1, antiPD-1 or antiCTLA4 individually. Additive tumor cell death was seen when antiCHI3L1 was administered in combination with antiPD-1 or and CTLA4 alone. Most importantly, highly impressive synergistic tumor cell death was seen when the cocultures were treated with the bispecific antibodies (FRGxCTLA4 or FRGxPD-1). In all cases the cell death that was induced was due to T cell differentiation into CD8+ cytotoxic T cells.
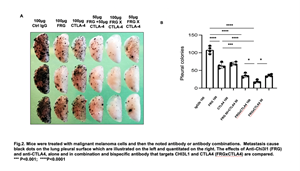
In the murine metastasis model we administered malignant melanoma cells into the murine circulation and evaluated their spread to the lungs and pleural surface by counting the number of black staining pleural metastasis. Tumor metastasis were readily appreciated in lungs from mice treated with isotype control antibodies, and modest decreases in metastasis were seen in lungs from mice treated with monospecific antiCHI3L1, antiPD-1 or antiCTLA4 individually. Additive inhibition of tumor spread was seen when antiCHI3L1 was administered in combination with antiPD-1 or and CTLA4. Most importantly, highly impressive synergistic inhibition of tumor metastasis was seen in lungs from mice treated with the bispecific antibodies (FRGxCTLA4 or FRGxPD-1). Figure 2 below shows the results with the FRGxCTLA4 antibody.
Quoted
“Bispecific antibodies that simultaneously target CHI3L1 and ICPI like PD-1 and or CTLA4 have an impressive and synergic ability to induce tumor cell death and prevent tumor metastasis compared to individual antibody moieties,” commented Dr. Jack A. Elias, Dean Emeritus of Medicine and Biological Sciences and Professor of Translational Science, Medicine and Molecular Microbiology and Immunology at the Warren Alpert Medical School Brown University; Scientific co-founder.
“Non-small cell lung cancer (NSCLC) is the leading cause of cancer death and second most diagnosed cancer in the US. Glioblastoma multiforme (GBM) is a lethal type of brain tumor that affects approximately 28,000 people in the U.S. The median survival time is about 15 months. With our discovery that CHI3L1 is a critical regulator of a number of key cancer-causing pathways by highlighting its ability to inhibit tumor cell death (apoptosis) this therapy has the potential to save thousand of lives of people effected from NSCLC and GSM,” said Dr. Chirinjeev Kathuria, co-founder and Executive Chairman.
Suren Ajjarapu, Chairman and CEO of Aesther, commented, “Aesther is honored to be part of the exciting discovery announced by Ocean Biomedical today. We look forward to working with Ocean to bring these therapies to patients. This discovery and others will lead to long term shareholder value growth and appreciation.”
About Aesther Healthcare Acquisitions Corp.
Aesther is a special purpose acquisition company (SPAC) formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. Its principals possess public and private market investing experience and operational knowledge to bring value added benefits to Ocean Biomedical. The Aesther team has substantial experience investing in and operating businesses in multiple sectors, as well as a significant long-term track record in creatively structuring transactions to unlock and maximize value.
To learn more, visit www.aestherhealthcarespac.com.
About Ocean Biomedical
Ocean Biomedical, Inc. is a Providence, Rhode Island-based biopharma company with an innovative business model that accelerates the development and commercialization of scientifically compelling assets from research universities and medical centers. Ocean Biomedical deploys the funding and expertise to move new therapeutic candidates efficiently from the laboratory to the clinic, to the world. Ocean Biomedical is currently developing five promising discoveries that have the potential to achieve life-changing outcomes in lung cancer, brain cancer, pulmonary fibrosis, and the prevention and treatment of malaria. The Ocean Biomedical team is working on solving some of the world’s toughest problems, for the people who need it most.
To learn more, visit www.oceanbiomedical.com
Forward-Looking Statements
This press release contains certain statements that are not historical facts and are forward-looking statements within the meaning of the federal securities laws with respect to the proposed merger agreement between Aesther and Ocean Biomedical (the “Transaction”), including without limitation statements regarding the anticipated benefits of the proposed Transaction, the anticipated timing of the proposed Transaction, the implied enterprise value, future financial condition and performance of Ocean Biomedical and the combined company after the closing and expected financial impacts of the proposed Transaction, the satisfaction of closing conditions to the proposed Transaction, the level of redemptions of Aesther's public stockholders and the products and markets and expected future performance and market opportunities of Ocean Biomedical. These forward-looking statements generally are identified by the words "believe," "project," "expect," "anticipate," "estimate," "intend," “think,” "strategy," "future," "opportunity," “potential,” "plan," “seeks,” "may," "should," "will," "would," "will be," "will continue," "will likely result," and similar expressions, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties.
The announcement today is based solely on laboratory and animal studies. Ocean Biomedical has not conducted any studies that show similar efficacy or safety in humans. There can be no assurances that this treatment will prove safe or effective in humans, and that any clinical benefits of this treatment is subject to clinical trials and ultimate approval of its use in patients by the FDA. Such approval, if granted, could be years away.
These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many factors could cause actual future events to differ materially from the forward-looking statements in this communication, including but not limited to: (i) the risk that the proposed Transaction may not be completed in a timely manner or at all, which may adversely affect the price of Aesther's securities; (ii) the risk that the proposed Transaction may not be completed by Aesther's business combination deadline; (iii) the failure to satisfy the conditions to the consummation of the proposed Transaction, including the approval of the Merger Agreement by the stockholders of Aesther, the satisfaction of the minimum net tangible assets and minimum cash at closing requirements and the receipt of certain governmental, regulatory and third party approvals; (iv) the occurrence of any event, change or other circumstance that could give rise to the termination of the Merger Agreement; (v) the failure to achieve the minimum amount of cash available following any redemptions by Aesther's stockholders; (vi) redemptions exceeding anticipated levels or the failure to meet The Nasdaq Global Market's initial listing standards in connection with the consummation of the proposed Transaction; (vii) the effect of the announcement or pendency of the proposed Transaction on Ocean Biomedical’s business relationships, operating results, and business generally; (viii) risks that the proposed Transaction disrupts current plans and operations of Ocean Biomedical; (ix) the outcome of any legal proceedings that may be instituted against Ocean Biomedical or against Aesther related to the Merger Agreement or the proposed Transaction ; (x) changes in the markets in which Ocean Biomedical’s competes, including with respect to its competitive landscape, technology evolution, or regulatory changes; (xi) changes in domestic and global general economic conditions; (xii) risk that Ocean Biomedical may not be able to execute its growth strategies; (xiii) risks related to the ongoing COVID-19 pandemic and response, including supply chain disruptions; (xiv) risk that Ocean Biomedical may not be able to develop and maintain effective internal controls; (xv) costs related to the proposed Transaction and the failure to realize anticipated benefits of the proposed Transaction or to realize estimated pro forma results and underlying assumptions, including with respect to estimated stockholder redemptions; (xvi) the ability to recognize the anticipated benefits of the proposed Transaction and to achieve its commercialization and development plans, and identify and realize additional opportunities, which may be affected by, among other things, competition, the ability of Ocean Biomedical to grow and manage growth economically and hire and retain key employees; (xvii) the risk that Ocean Biomedical may fail to keep pace with rapid technological developments to provide new and innovative products and services or make substantial investments in unsuccessful new products and services; (xviii) the ability to develop, license or acquire new therapeutics; (xix) the risk that Ocean Biomedical will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; (xx) the risk that Ocean Biomedical, post-combination, experiences difficulties in managing its growth and expanding operations; (xxi) the risk of product liability or regulatory lawsuits or proceedings relating to Ocean Biomedical’s business; (xxii) the risk of cyber security or foreign exchange losses; (xxiii) the risk that Ocean Biomedical is unable to secure or protect its intellectual property; and (xxiv) those factors discussed in Aesther's filings with the SEC and that that will be contained in the proxy statement relating to the proposed Transaction .
The foregoing list of factors is not exhaustive. You should carefully consider the foregoing factors and the other risks and uncertainties that will be described in the "Risk Factors" section of the preliminary proxy statement and the amendments thereto, the definitive proxy statement, and other documents to be filed by Aesther from time to time with the SEC. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and while Ocean Biomedical and Aesther may elect to update these forward-looking statements at some point in the future, they assume no obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. Neither of Ocean Biomedical or Aesther gives any assurance that Ocean Biomedical or Aesther, or the combined company, will achieve its expectations. These forward-looking statements should not be relied upon as representing Aesther’s or Ocean Biomedical’s assessments as of any date subsequent to the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Additional Information and Where to Find It
In connection with the merger agreement and the proposed Transaction, Aesther intends to file with the U.S. Securities and Exchange Commission (the “SEC”) a proxy statement on Schedule 14A relating to the proposed Transaction. This communication is not intended to be, and is not, a substitute for the proxy statement or any other document that Aesther has filed or may file with the SEC in connection with the proposed Transaction. Aesther’s stockholders and other interested persons are advised to read, when available, the preliminary proxy statement and the amendments thereto, the definitive proxy statement and documents incorporated by reference therein filed in connection with the proposed Transaction, as these materials will contain important information about Aesther, Ocean Biomedical, the merger agreement, and the proposed Transaction. When available, the definitive proxy statement and other relevant materials for the proposed Transaction will be mailed to stockholders of Aesther as of a record date to be established for voting on the proposed Transaction. Before making any voting or investment decision, investors and stockholders of Aesther are urged to carefully read the entire proxy statement, when they become available, and any other relevant documents filed with the SEC, as well as any amendments or supplements to these documents, because they will contain important information about the proposed Transaction. Aesther investors and stockholders will also be able to obtain copies of the preliminary proxy statement, the definitive proxy statement, and other documents filed with the SEC that will be incorporated by reference therein, without charge, once available, at the SEC’s website at www.sec.gov, or by directing a request to: Aesther Healthcare Acquisition Corp., 515 Madison Avenue, Suite 8078, New York, NY 10022, Attention: Mr. Suren Ajjarapu.
Participants in the Solicitation
Aesther, Ocean Biomedical and their respective directors, executive officers, other members of management and employees may be deemed participants in the solicitation of proxies from Aesther’s stockholders with respect to the proposed Transaction. Investors and security holders may obtain more detailed information regarding the names and interests in the proposed Transaction of Aesther’s directors and officers in Aesther’s filings with the SEC, including, when filed with the SEC, the preliminary proxy statement and the amendments thereto, the definitive proxy statement, and other documents filed with the SEC. Such information with respect to Ocean Biomedical’s directors and executive officers will also be included in the proxy statement.
No Offer or Solicitation
This press release is not a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed Transaction and will not constitute an offer to sell or the solicitation of an offer to buy any securities, nor will there be any sale of securities in any states or jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction.
# # #
Investor Contact
IR@aestherhealthcarespac.com
Ocean Biomedical Media Relations
Kevin Kertscher
Communications Director
kkertscher@oceanbiomedical.com
Attachments