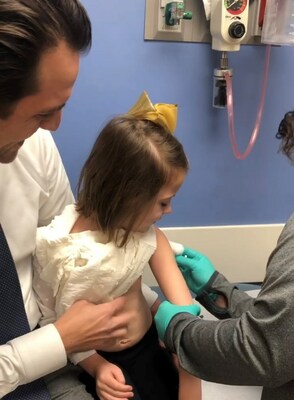
Live vaccinations provided to children who previously received liver or kidney transplants were found to be safe and prompted an immune response to guard against several life-threatening conditions, according to a new study published Oct. 12, 2023, in JAMA Network Open.
|
|
| [16-October-2023] |
|
Experts at Cincinnati Children's and Children's Hospital Colorado say findings could enable more at-risk children to be protected from measles, mumps, and chicken pox CINCINNATI, Oct. 16, 2023 /PRNewswire/ -- Live vaccinations provided to children who previously received liver or kidney transplants were found to be safe and prompted an immune response to guard against several life-threatening conditions, according to a new study published Oct. 12, 2023, in JAMA Network Open. The study, based on data from 18 organ transplant centers, was co-authored by Lara Danziger-Isakov, MD, MPH, interim director of the Division of Infectious Diseases at Cincinnati Children's, and Amy Feldman, MD, MSCS, medical director of the Liver Transplant Program at Children's Hospital Colorado. The results are important because the rates of measles, mumps and the varicella-zoster virus that causes chicken pox are rising nationally and internationally, leaving immunocompromised children at risk for life-threatening conditions, according to Danziger-Isakov, senior author of the study. "This shifts the paradigm of the approach to protecting this vulnerable population where live viral vaccines were previously avoided," Danziger-Isakov says. "This should enable children who have received organ transplants to integrate into their communities with more confidence and decreased risk for acquiring chicken pox or measles, which have both made a resurgence." Global surges of measles and mumps were exacerbated by decreasing herd immunity as millions of vaccine doses were missed during the COVID-19 pandemic, leaving nonimmune organ transplant recipients at significant risk for community exposure, infection, disease and death from wild-type infection, says Feldman, first author of the study. "Community acquisition of measles and varicella is a real risk for immunocompromised children in today's world," Feldman says. "These infections can be fatal in transplant recipients. Being able to administer live vaccines post-transplant, and provide immunity, is critical." Historically, live vaccines to guard against measles, mumps, rubella (MMR) and varicella-zoster virus (VZV) have not been recommended after solid organ transplant due to concerns about a theoretical risk of vaccine strain infection in immunocompromised children. However, the authors reported no serious adverse events observed following the live vaccination of the children enrolled in the trial between Jan. 1, 2002, and Feb. 28, 2023. Transplant recipients who participated in the trial received one to three doses of MMR vaccine and/or one to three doses of VZV vaccine. The cohort included 281 children on chronic immunosuppressive medications with a median age of 8.9 years at the time of the first post-transplant vaccine. The median time from transplant to enrollment in the study was 6.3 years. Safety data were collected after each vaccination, and antibody levels were measured at zero to three months and one year after vaccination. The majority of children developed protective antibodies following vaccination – 72% for varicella, 86% for measles, 83% for mumps and 99% for rubella. One year after vaccination, the majority of children who developed protective antibodies maintained that protection – 77% for varicella, 92% for measles, 83% for mumps and 94% for rubella. Five children developed clinical varicella, but all of their conditions resolved within one week. The findings suggest that live vaccinations may be safe and immunogenic after solid organ transplant in select pediatric recipients and can offer protection against circulating measles, mumps and varicella. Further research is needed to understand long-term maintenance of immunity after vaccination of children who receive organ transplants, as well as factors associated with immune response and clinical protection, the authors noted. Feldman reported that she is funded by a K08 grant (HS026510) from the Agency for Healthcare Research and Quality. Danziger-Isakov reported receiving clinical research support paid to her institution from AiCuris, Ansun Biopharma, Astellas Pharma, Merck, Pfizer and Takeda; advisory board fees from GlaxoSmithKline and Roche; and consultant fees from Merck outside the submitted work. About Cincinnati Children's Cincinnati Children's ranks No. 1 in the nation in U.S. News & World Report's 2023-24 listing of Best Children's Hospitals. In addition, Cincinnati Children's is recognized as one of America's Most Innovative Companies by Fortune. Nearly one-third of the health system's 18,500 employees are engaged in research, and Cincinnati Children's is one of the top two recipients of pediatric research grants from the National Institutes of Health. Additional information may be found at www.cincinnatichildrens.org
SOURCE Cincinnati Children's Hospital Medical Center |