Ultivue, a life science company developing reagent-driven strategies for high-performance biological imaging in situ, today announced expanded capabilities of its proprietary InSituPlex® DNA-barcoding and staining technology.
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Ultivue, a life science company developing reagent-driven strategies for high-performance biological imaging in situ, today announced expanded capabilities of its proprietary InSituPlex® DNA-barcoding and staining technology. InSituPlex® now supports a higher level of multiplexed detection and quantification of markers in tissue samples while retaining all the benefits of the company’s UltiMapper™ assays.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181107005973/en/
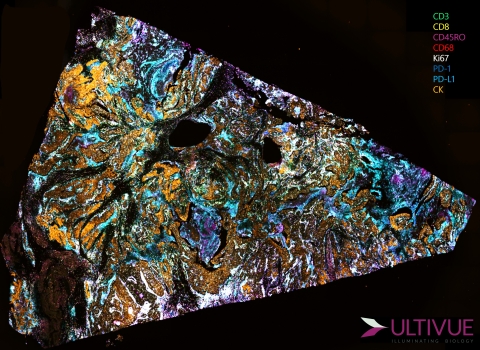
A whole-slide scan of a non-small cell lung cancer (NSCLC) tissue simultaneously stained with an 8-marker multiplex IHC panel using InSituPlex® technology and UltiMapper™ I/O assays. (Photo: Business Wire)
“We are delighted to introduce our 9-color/8-marker whole-slide, single staining step multiplexing assay which provides exceptional data quality and throughput, with sample-to-analysis workflow achieved in a single workday, using instrumentation and software solutions currently found in most immunohistochemistry laboratories,” said Philippe Mourere, Ultivue’s Senior Vice President of Commercial Operations. Data illustrating the assay workflow and performance will be presented in the poster sessions on Saturday, November 10th by Dr. Gourab Chatterjee, Scientist at Ultivue.
“Ultivue continues executing its strategic plan by delivering breakthrough innovations that fuel our portfolios of Products and Services, making tissue multiplexing a realized assay from exploratory discovery to translational and clinical research,” added Mourere.
Ultivue’s scientific team is also presenting 5 high impact posters at the upcoming SITC Annual Meeting, at the Walter E. Washington Convention Center in Washington, D.C., from November 7 to 11, 2018. Data will showcase the performance of two new UltiMapper™ I/O research kits for the detection and quantitation of Antigen Presenting Cells (APC), and the characterization of T-cell activation (T-act) in tumor tissue samples. Ultivue’s APC kit (CD11c, CD20, CD68/CD163, MHC II) and T-act kit (CD3, GranzymeB, Ki67, CK/Sox10) synergistically complement the existing UltiMapper™ I/O PD-L1 and PD-1 kits for the holistic immuno-profiling of the tumor microenvironment.
“We will also present new data on the expansion of the automated UltiMapper™ protocol and workflows, as well as data illustrating differentiated PD-L1 expression levels on multiple cell types,” said Karan Sharma, Product Manager at Ultivue. “Additionally, InSituPlex® technology improvements, and robust assay verification data will be presented as evidence of exceptional assay performance and reproducibility in a wide range of FPPE tissue samples.”
Ultivue’s team will be available to engage with the SITC community and discuss your current and future research projects at booth #625, throughout the duration of the conference.
Our scientists will be presenting at the following poster numbers and dates:
Friday, November 9, 12:45 PM-2:15 PM & 6:30 PM-8 PM
• P39: PD-L1 expression on tumor versus antigen presenting cells investigated with multiplexed IHC using UltiMapper™ I/O assays.
• P627: From staining to analysis: fully automated workflow for multiplexed immuno-profiling in FFPE tumor samples using UltiMapper™ reagent kits.
Saturday, November 10, 12:20 PM-1:50 PM & 7 PM-8:30 PM
• P38: Highly multiplex spatial immuno-profiling in FFPE tumor tissue with InSituPlex® technology.
• P40: Intra-assay and inter-assay assessment of reproducibility and quantification of UltiMapper I/O PD-1 and PD-L1 immuno-oncology panels for tissue multiplexing.
• P654: T-cell Profiling in cancer tissue with multiplexed IHC using UltiMapper™ I/O assays.
About Ultivue
Multiplexed biomarker assays in tissue for personalized medicine research and digital pathology.
By developing a single set of novel, proprietary reagents used both for biomarker discovery (higher content, low throughput) and translational use (lower content, high throughput), Ultivue is connecting the insights gained from research directly into the pathology lab. Ultivue’s UltiMapper™ multiplexed assays applied to tissue biopsy samples enable simultaneous quantitation of multiple biomarkers with sub-cellular spatial resolution and fit completely within traditional IHC workflows. Translational and clinical researchers leverage UltiMapper™ assays to elucidate complex biology and demonstrate their clinical utility as precision medicine research tools. Ultivue is expanding its UltiMapper™ assay product portfolio and menu of contract research services to provide a comprehensive set of personalized medicine solutions for oncology research and focus in other therapeutic areas.
Ultivue is based in Cambridge, MA. For more information, visit www.ultivue.com
View source version on businesswire.com: https://www.businesswire.com/news/home/20181107005973/en/
Source: Ultivue Inc.
Smart Multimedia Gallery
A whole-slide scan of a non-small cell lung cancer (NSCLC) tissue simultaneously stained with an 8-marker multiplex IHC panel using InSituPlex® technology and UltiMapper™ I/O assays. (Photo: Business Wire)






