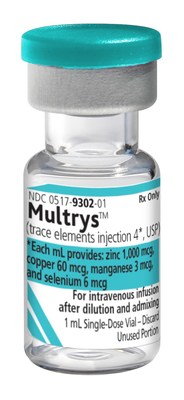
American Regent, Inc. introduces FDA-approved Multrys™ (trace elements injection 4*, USP).
|
MELVILLE, N.Y., Sept. 2, 2021 /PRNewswire/ -- American Regent, Inc. introduces FDA-approved Multrys™ (trace elements injection 4*, USP). Multrys™ is indicated in neonatal and pediatric patients weighing less than 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.2 View more product specific information. "We are pleased to offer another FDA-approved multiple trace elements injection specifically developed to more closely align with the American Society for Parenteral and Enteral Nutrition (ASPEN) recommendations for trace element supplementation than previously marketed products.3 This new formulation, which is manufactured in America, has been designed to meet the special needs of the neonatal and pediatric patient population and is part of our overall initiative to retire our line of marketed unapproved trace element products," stated Joann Gioia, Vice President and Chief Commercial Officer at American Regent, Inc. "The launch of Multrys™ demonstrates American Regent's continued commitment to addressing the needs of patients who require trace element supplementation." This product is available for immediate shipment. Customers can order Multrys™ through their wholesaler/distributor, or by contacting our Customer Support Group at 1-800-645-1706. Multrys™ (trace elements injection 4*, USP) is supplied as follows:
Please see the Important Safety Information below. To view the Full Prescribing Information, please click here. For additional information on Multrys visit www.americanregent.com. References PP-MU-US-0012 8/2021 Multrys ™ (trace elements injection 4*, USP) For intravenous use INDICATIONS AND USAGE Multrys is a combination of trace elements (zinc sulfate, cupric sulfate, manganese sulfate, and selenious acid) indicated in neonatal and pediatric patients weighing less than 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. IMPORTANT SAFETY INFORMATION DOSAGE AND ADMINISTRATION Overview of Dosing CONTRAINDICATIONS WARNINGS AND PRECAUTIONS Vein Damage and Thrombosis: Solution with an osmolarity of 900 mOsmol/L or greater must be infused through a central catheter. Neurologic Toxicity with Manganese: Monitor for clinical signs and symptoms of neurotoxicity, whole blood manganese concentrations, and liver function tests. Discontinue Multrys and consider brain magnetic resonance imaging (MRI) if toxicity is suspected. Monitor patients for cholestasis or other biliary liver disease. Hepatic Accumulation of Copper and Manganese: Assess for development of hepatic accumulation. Monitor concentrations of copper and manganese in patients with cholestasis or cirrhosis. Aluminum Toxicity: Multrys contains aluminum that may be toxic. Patients with renal impairment and preterm infants, including preterm neonates, are particularly at risk. Monitoring and Laboratory Tests: Monitor blood zinc, copper and selenium serum concentrations, whole blood manganese concentration, fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count, and coagulation parameters. Hypersensitivity Reactions with Zinc and Copper: If hypersensitivity reactions occur, discontinue and initiate appropriate medical treatment. ADVERSE REACTIONS
OVERDOSAGE For additional safety information, please see Full Prescribing Information. You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236, or to the FDA by visiting www.fda.gov/medwatch or by calling 1-800-FDA-1088. REF-1826 6/2021 You are encouraged to report Adverse Drug Events (ADEs) to American Regent: Email: pv@americanregent.com; Fax: 1-610-650-0170; ADEs may also be reported to the FDA: Drug Information: For urgent drug information outside of normal business hours, About American Regent American Regent is committed to US based manufacturing. To that end, over the last several years, we have made significant investments in expanding and modernizing our manufacturing facilities in Ohio and New York. This expansion will nearly double our capacity and allow us to better serve our customers now and in the future. Speed counts. Flexibility matters. Reliability and quality are paramount. Because patients should never have to wait for the medications they need. For more information, please visit www.americanregent.com. About Daiichi Sankyo Group Daiichi Sankyo, Inc., headquartered in Basking Ridge, New Jersey, is a member of the Daiichi Sankyo Group. For more information on Daiichi Sankyo, Inc., please visit: www.dsi.com
SOURCE American Regent, Inc. |