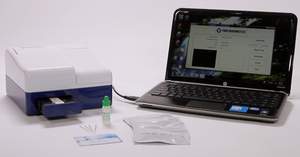
SAN DIEGO, CA--(Marketwire - May 24, 2012) - True Diagnostics, Inc.™ (www.TrueDiagnostics.com) has announced it has received a CE Mark to allow it to distribute its TrueDX Platform and quantitative TSH (Thyroid Stimulating Hormone) test in Europe. With the TrueDX Platform, doctors can immediately determine if a patient suffers from a hypoactive thyroid by using only a finger prick of blood. This unique test enables doctors and patients to gain access to diagnosis in minutes, create an effective treatment plan, while saving physicians' time and patients cost and anxiety. True Diagnostics is actively engaging distribution partners throughout all EU markets, with initial focus in Germany, UK, France, Italy and Spain.
This is the second regulatory clearance for the TrueDX Platform with TSH test, having received sFDA approval for China in September 2011. True Diagnostics expects to run short clinical studies in the U.S. this summer and further submit for CLIA-waive and 510k FDA clearance to market the TrueDX Platform with quantitative TrueDX-TSH™ Tests throughout the U.S.
What will the TrueDX Platform mean for doctors and patients seeking to more easily identify Hypoactive Thyroid? Hypothyroidism affects over 200 million people globally, more than people with diabetes. TSH is the fourth most requested blood test in America. Hypothyroidism is commonly undiagnosed and misdiagnosed with symptoms ranging from weight gain to depression, forgetfulness, hair loss, thinning and drying of skin, fatigue, loss of libido, feeling cold, constipation, and a pronounced goiter.
Jerry Lee, President/CEO of True Diagnostics, notes, "With this latest regulatory clearance, the Company has laid the foundation to begin standardizing a full range of diagnostic and monitoring tests that will provide instant results to doctors and patients and demand little training. With distribution partners already lined up and more coming soon, we expect initial shipments to Europe to begin within 60 days."
The strength of the TrueDX Platform lies in its simplicity. Tests have a 24-month shelf life. They do not need to be refrigerated. Laboratory quality results from small samples can now be available to doctors to review in minutes. The TrueDX Platform's simplicity, portability, and affordability allow it to be easily adopted into emerging markets. The platform's flexible design also permits it to stay ahead of potential competitors and quickly commercialize a myriad of new biomarkers discoveries for cancer, autism, alzheimer's, diabetes, drugs of abuse, infectious diseases, cardiac health, traumatic brain injury, viral and bacterial infections, and more. In essence, any biomarker discovery utilizing traditional lab methods, such as an ELISA process, can now be developed into a simple, fast, and portable assay which can offer quick diagnosis and treatment right in the physician's office.
About the Company
True Diagnostics, Inc. creates simple diagnostic solutions for the $46 billion medical point-of-care, sterility assurance, animal health, and food processing markets. After 30 years in the global medical diagnostic field, the inventor of the home pregnancy test created the TrueDX Platform which can measure the severity of any medical condition (using only a finger prick of blood, small urine or saliva sample, toxins, surface bacteria, and more), provide results in minutes (not days), and is completely portable. The TrueDX Platform takes point-of-care, point-of-incidence, and remote-of-care applications out of the lab to provide more flexible treatment that can begin immediately. The TrueDX Platform creates unique and powerful opportunities for new diagnostic solutions through its ability to: 1) quickly bring new diagnostic tests to market; and, 2) open new geographic and application opportunities.
For more information, call 760-683-9158 or go to www.TrueDiagnostics.com