PLANO, TX, April 11, 2017 /PRNewswire/ - Texas Back Institute continues to lead the way in spine surgery by being the first in the US to use the AnchorKnot® Tissue Approximation Kit, a novel technology for use in herniated disc repair.
Dr. Scott L. Blumenthal, board-certified orthopedic spine surgeon, physician executive with Texas Back Institute and Co-Medical Director of Center for Disc Replacement, performed a discectomy procedure on a young patient in February 2017. This patient was the first in the US to undergo herniated disc repair using the AnchorKnot® Kit.
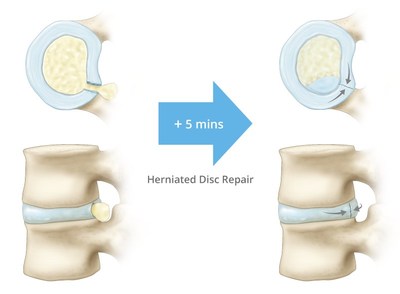
Lumbar disc herniation is one of the most common causes for back surgery, and can occur from injury or disc degeneration. In both cases, material from a damaged disc presses on the spinal nerve, causing many patients to experience pain, which can significantly decrease their quality of life. A standard discectomy procedure offers effective relief; however, it commonly results in a defect that until now, has been difficult to repair without specific tools. Studies have shown that 10-15% of patients could suffer a reherniation through this defect.
The Texas Back Institute completed nearly 500 discectomy cases in 2016. Dr. Blumenthal comments that "most discectomy procedures are left to heal through the body's scar tissue which could take 6 weeks or more, so if approximating the tissue can return patients to function and to rehab earlier then this could be a real benefit to patients." Dr. Blumenthal indicates that there has been literature and commercial attempts in the past and "with appropriate studies and follow-up this could be a game changer for patient outcomes."
"As an experienced medical device company, we have worked closely for numerous years with surgeons to design the AnchorKnot® Tissue Approximation Kit for use in herniated disc repair. It is a tremendous honor for Anchor Orthopedics to have our first US clinical partner at the Texas Back Institute," said Neil Godara, General Manager. "Our goal is to design clinically meaningful products that improve the lives of patients around the world. We look forward to offering the AnchorKnot® Tissue Approximation Kit to patients in need of a discectomy procedure, and continuing to collaborate with Dr. Blumenthal and his team at the Texas Back Institute."
About Anchor Orthopedics
Anchor Orthopedics XT Inc., the developer of the AnchorKnot® Tissue Approximation Kit, aims to provide surgeons with novel solutions that optimize procedures in disc repair in an effort to preserve the biomechanics of the patient and improve surgical outcomes. Our mission is to work closely with surgeons on all aspects of development to create clinical solutions that serve to improve the lives of patients around the world. Anchor Orthopedics XT Inc., located in Mississauga, Canada, is a subsidiary of Baylis Medical Company Inc.
PRM-00194 EN J-1 V-1 © Anchor Orthopedics XT Inc., 2017. Anchor Orthopedics XT and AnchorKnot are trademarks and/or registered trademarks of Anchor Orthopedics XT Inc. in the USA and/or other countries. All other trademarks are the property of their respective owners. CAUTION: Federal Law (USA) restricts the use of these devices to or by the order of a physician. Before use, consult product labels and Instructions for Use for Indications for Use, Contraindications, Warnings, Precautions, Adverse Events and Directions for Use. The AnchorKnot Suture Passer (2-0) is an accessory of the Anchor System. The Anchor System is indicated for visualization of the surgical field in any area of the body cut open during a surgical procedure. When used in the cervical, thoracic, or lumbar spine either from an anterior or posterior direction, for example, the Anchor Endoscope and accessories are intended to aid the surgeon's visualization of the surgical area and allow him/her to perform any type of surgical spinal procedure such as herniated disc repair, visualization of the circumferential decompression of the nerve roots, aiding in the search and removal of nucleus material, spinal fusion, or insertion of spinal implants. Other examples of generic surgical use of the Anchor System would be for use in the knee, ankle, shoulder, hand, wrist, and temporomandibular joint (TMJ). Patents pending and/or issued.
Media Contact Person:
Ester Kwok
Global Product Manager
ekwok@anchorortho.com
Tel: 647-484-0662 ext 388
Anchor Orthopedics XT Inc.
http://www.anchorortho.com

SOURCE Anchor Orthopedics





