Renal Cell Carcinoma Market Outlook 2024-2034:
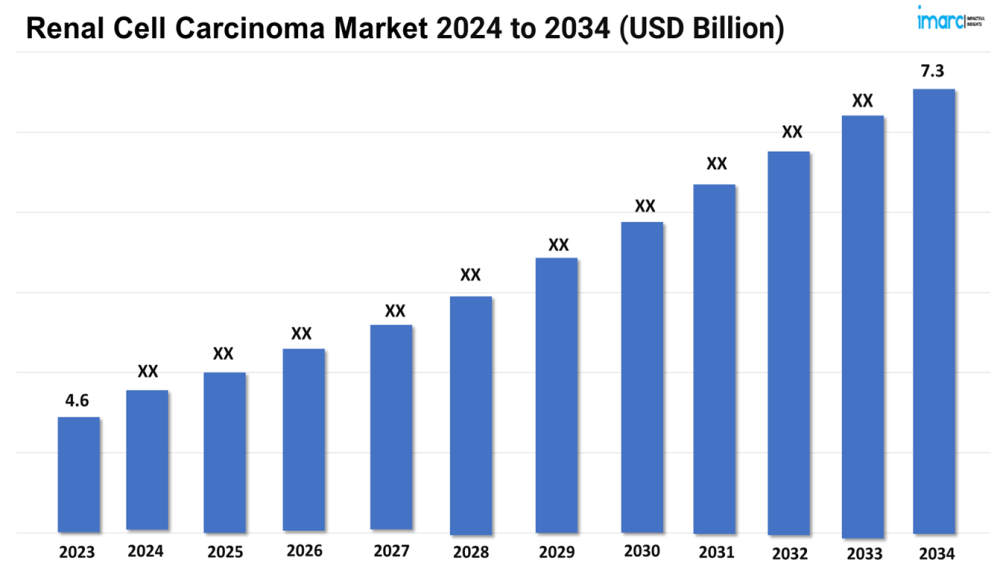
The renal cell carcinoma market size reached a value of USD 4.6 Billion in 2023. Looking forward, the market is expected to reach USD 7.3 Billion by 2034, exhibiting a growth rate (CAGR) of 4.22% during 2024-2034.
The market is driven by numerous advancements in targeted therapies and immunotherapies. Increased focus on personalized medicine, rising incidence rates, and ongoing clinical trials are driving innovation. Additionally, collaborations between pharmaceutical companies and research institutions are accelerating the development of novel treatment options.
Advancements in Targeted Therapies and Immunotherapies: Driving the Renal Cell Carcinoma Market
Recent advancements in targeted therapies and immunotherapies are transforming the renal cell carcinoma (RCC) market, offering patients innovative and more effective treatment options. Targeted therapies have revolutionized RCC management by focusing on specific molecular targets that are crucial for tumor growth and survival. Tyrosine kinase inhibitors (TKIs) like sunitinib, pazopanib, and axitinib have become pivotal in RCC treatment. These drugs inhibit pathways such as the vascular endothelial growth factor (VEGF) receptor pathway, which is essential for tumor angiogenesis. By disrupting this pathway, TKIs effectively limit the blood supply to tumors, thereby inhibiting their growth. Newer agents, such as cabozantinib and lenvatinib, are further expanding the therapeutic arsenal, offering additional options for patients who have developed resistance to earlier treatments.
Request a PDF Sample Report: https://www.imarcgroup.com/renal-cell-carcinoma-market/requestsample
Immunotherapy has also made substantial strides in the RCC market. Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, have been groundbreaking. These drugs work by blocking proteins that prevent immune cells from attacking cancer cells. This approach enhances the body's immune response against tumors and has proven effective in treating metastatic RCC. The combination of immunotherapy with targeted therapies, such as the pairing of nivolumab with cabozantinib, has shown synergistic effects, improving overall survival and response rates. Additionally, ongoing research and clinical trials are exploring novel combinations and new therapeutic targets, further enhancing treatment options. Personalized medicine, driven by advancements in biomarker identification, is allowing for tailored treatment strategies that optimize efficacy and reduce side effects. These developments are collectively advancing RCC treatment, providing new hope for patients and setting the stage for even more effective therapies in the future.
Increased Focus on Personalized Medicine: Contributing to Market Expansion
The increased focus on personalized medicine is driving significant advancements in the renal cell carcinoma market, revolutionizing treatment approaches and enhancing patient outcomes. Personalized medicine aims to tailor treatments based on individual patient characteristics, including genetic, molecular, and environmental factors. This approach contrasts with the traditional one-size-fits-all model and has proven particularly effective in managing complex cancers like RCC. In RCC, personalized medicine starts with the identification of specific genetic mutations and molecular markers associated with tumor growth and progression. This has led to the development of targeted therapies that address these unique features. For example, the use of genetic profiling to identify mutations in the VEGF and mTOR pathways has enabled the development of targeted drugs such as sunitinib, pazopanib, and everolimus. These therapies specifically inhibit the pathways involved in tumor growth, leading to more effective and less toxic treatments compared to conventional options.
Moreover, the integration of genomic data into treatment planning allows for a more precise selection of therapies. Biomarker-driven approaches enable oncologists to predict which patients are more likely to respond to specific treatments, thus optimizing therapeutic efficacy and minimizing unnecessary side effects. For instance, patients with high PD-L1 expression may benefit more from immune checkpoint inhibitors like nivolumab and pembrolizumab, which enhance the body's immune response against cancer cells. Additionally, personalized medicine facilitates the development of combination therapies, where targeted agents are used in conjunction with immunotherapies to enhance overall treatment effectiveness. Ongoing research and clinical trials are continually expanding the repertoire of biomarkers and refining treatment algorithms to provide even more customized care. In summary, the increased focus on personalized medicine in the RCC market is leading to more precise, effective, and individualized treatment strategies. By tailoring therapies to the unique characteristics of each patient’s cancer, personalized medicine is setting new standards in RCC management and improving outcomes.
Ongoing Clinical Trials and Research:
Ongoing clinical trials and research are pivotal in advancing the renal cell carcinoma market, driving innovation and expanding treatment options for patients. One of the significant areas of focus in current research is the development of combination therapies. Researchers are investigating the synergistic effects of combining targeted therapies, such as tyrosine kinase inhibitors (TKIs), with immune checkpoint inhibitors. For example, trials exploring the combination of cabozantinib with nivolumab have shown promising results in improving overall survival rates and response rates compared to monotherapy. These studies aim to enhance treatment outcomes by leveraging the strengths of different therapeutic modalities. Another key area of research is the exploration of novel drug candidates and therapeutic targets. Clinical trials are evaluating new agents that target various pathways involved in RCC progression, such as the mTOR pathway and angiogenesis. Agents like belzutifan and avo-mab are being tested for their potential to provide additional treatment options for patients, especially those who have developed resistance to existing therapies.
Personalized medicine is also a significant focus in ongoing research. Clinical trials are investigating how genetic and molecular profiling can guide treatment decisions, allowing for more tailored and effective therapies. Biomarker-driven studies aim to identify which patients are most likely to benefit from specific drugs, thereby optimizing treatment strategies and minimizing adverse effects. Additionally, research into improving the quality of life for RCC patients is gaining traction. Trials assess supportive care interventions and strategies to manage treatment-related side effects, ensuring that patients receive comprehensive care throughout their treatment journey. In summary, ongoing clinical trials and research are crucial in shaping the future of RCC treatment. By exploring new drug combinations, novel targets, and personalized approaches, these efforts are expanding the therapeutic arsenal and offering new hope for patients battling renal cell carcinoma.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7313&method=587
Leading Companies in the Renal Cell Carcinoma Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global renal cell carcinoma market, several notable companies are actively pursuing new therapies and treatment strategies. This includes expanding clinical trials and investing in research to better understand RCC biology and improve treatment options. SillaJen Biotherapeutics and CoImmune have been investing heavily in their manufacturing capacities in recent months.
SillaJen submitted CSR to the US FDA on February 6, 2024, for REN026, a phase 1b/2a dose escalation and safety/efficacy evaluation study of Pexa-Vec in conjunction with cemiplimab in patients with metastatic or unresectable renal cell carcinoma.
Additionally, in November 2023, the FDA granted batiraxcept a fast-track designation (FTD) for patients with clear cell renal cell carcinoma who have progressed after receiving one or two previous courses of systemic therapy.
Apart from this, CoImmune published a review of its clinical development program for CMN-001, a dendritic cell-based immunotherapy electroporated with autologous tumor RNA to treat metastatic renal cell carcinoma.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7313&flag=E
Regional Analysis:
The major markets for renal cell carcinoma include the United States, Germany, France, the United Kingdom, Italy, Spain and Japan. According to projections by IMARC, the United States has the largest patient pool for renal cell carcinoma while also representing the biggest market for its treatment. This can be attributed to the growing emphasis on exploring various combination and sequential therapy approaches to improve treatment outcomes.
Moreover, the adoption of digital health technologies, including electronic health records, telemedicine, and remote monitoring tools, is improving patient management and care in RCC. These technologies enhance the ability to track patient progress, manage side effects, and ensure adherence to treatment regimens.
Apart from this, the emerging trend towards combining immunotherapies with targeted therapies, such as TKIs, is gaining momentum. Clinical trials and real-world studies are increasingly exploring combinations like nivolumab with cabozantinib, aiming to enhance efficacy and improve patient outcomes.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
This report offers a comprehensive analysis of current renal cell carcinoma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
IMARC Group Offer Other Reports:
Blood Pressure Monitoring Devices Market: The global blood pressure monitoring devices market size reached US$ 2.7 Billion in 2023, and projected to reach US$ 7.4 Billion by 2032, exhibiting a growth rate (CAGR) of 11.5% during the forecast period from 2024 to 2032.
Medical Device Security Market: The global medical device security market size reached US$ 9.7 Billion in 2023, and projected to reach US$ 30.6 Billion by 2032, exhibiting a growth rate (CAGR) of 13.18% during the forecast period from 2024 to 2032.
Compression Therapy Market: The global compression therapy market size reached US$ 3.9 Billion in 2023, and projected to reach US$ 6.4 Billion by 2032, exhibiting a growth rate (CAGR) of 5.48% during the forecast period from 2024 to 2032.
Glioblastoma Multiforme Treatment Market: The global glioblastoma multiforme treatment market size reached US$ 2.1 Billion in 2023, and projected to reach US$ 3.9 Billion by 2032, exhibiting a growth rate (CAGR) of 6.7% during the forecast period from 2024 to 2032.
Drug Discovery Informatics Market: The global drug discovery informatics market size reached US$ 3.3 Billion in 2023, and projected to reach US$ 7.8 Billion by 2032, exhibiting a growth rate (CAGR) of 9.7% during the forecast period from 2024 to 2032.
Ultraviolet Disinfection Equipment Market: The global ultraviolet (UV) disinfection equipment market size reached US$ 3.1 Billion in 2023, and expected to reach US$ 10.6 Billion by 2032, exhibiting a growth rate (CAGR) of 14.3% during the forecast period from 2024 to 2032.
Benign Prostatic Hyperplasia Treatment Market: The global benign prostatic hyperplasia treatment market size reached US$ 11.9 Billion in 2023, and expected to reach US$ 18.1 Billion by 2032, exhibiting a growth rate (CAGR) of 4.6% during the forecast period from 2024 to 2032.
Cannabis Infused Edible Products Market: The global cannabis infused edible products market size reached US$ 19.5 Billion in 2023, and projected to reach US$ 63.2 Billion by 2032, exhibiting a growth rate (CAGR) of 13.97% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The renal cell carcinoma market size reached a value of USD 4.6 Billion in 2023. Looking forward, the market is expected to reach USD 7.3 Billion by 2034, exhibiting a growth rate (CAGR) of 4.22% during 2024-2034.
The market is driven by numerous advancements in targeted therapies and immunotherapies. Increased focus on personalized medicine, rising incidence rates, and ongoing clinical trials are driving innovation. Additionally, collaborations between pharmaceutical companies and research institutions are accelerating the development of novel treatment options.
Advancements in Targeted Therapies and Immunotherapies: Driving the Renal Cell Carcinoma Market
Recent advancements in targeted therapies and immunotherapies are transforming the renal cell carcinoma (RCC) market, offering patients innovative and more effective treatment options. Targeted therapies have revolutionized RCC management by focusing on specific molecular targets that are crucial for tumor growth and survival. Tyrosine kinase inhibitors (TKIs) like sunitinib, pazopanib, and axitinib have become pivotal in RCC treatment. These drugs inhibit pathways such as the vascular endothelial growth factor (VEGF) receptor pathway, which is essential for tumor angiogenesis. By disrupting this pathway, TKIs effectively limit the blood supply to tumors, thereby inhibiting their growth. Newer agents, such as cabozantinib and lenvatinib, are further expanding the therapeutic arsenal, offering additional options for patients who have developed resistance to earlier treatments.
Request a PDF Sample Report: https://www.imarcgroup.com/renal-cell-carcinoma-market/requestsample
Immunotherapy has also made substantial strides in the RCC market. Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, have been groundbreaking. These drugs work by blocking proteins that prevent immune cells from attacking cancer cells. This approach enhances the body's immune response against tumors and has proven effective in treating metastatic RCC. The combination of immunotherapy with targeted therapies, such as the pairing of nivolumab with cabozantinib, has shown synergistic effects, improving overall survival and response rates. Additionally, ongoing research and clinical trials are exploring novel combinations and new therapeutic targets, further enhancing treatment options. Personalized medicine, driven by advancements in biomarker identification, is allowing for tailored treatment strategies that optimize efficacy and reduce side effects. These developments are collectively advancing RCC treatment, providing new hope for patients and setting the stage for even more effective therapies in the future.
Increased Focus on Personalized Medicine: Contributing to Market Expansion
The increased focus on personalized medicine is driving significant advancements in the renal cell carcinoma market, revolutionizing treatment approaches and enhancing patient outcomes. Personalized medicine aims to tailor treatments based on individual patient characteristics, including genetic, molecular, and environmental factors. This approach contrasts with the traditional one-size-fits-all model and has proven particularly effective in managing complex cancers like RCC. In RCC, personalized medicine starts with the identification of specific genetic mutations and molecular markers associated with tumor growth and progression. This has led to the development of targeted therapies that address these unique features. For example, the use of genetic profiling to identify mutations in the VEGF and mTOR pathways has enabled the development of targeted drugs such as sunitinib, pazopanib, and everolimus. These therapies specifically inhibit the pathways involved in tumor growth, leading to more effective and less toxic treatments compared to conventional options.
Moreover, the integration of genomic data into treatment planning allows for a more precise selection of therapies. Biomarker-driven approaches enable oncologists to predict which patients are more likely to respond to specific treatments, thus optimizing therapeutic efficacy and minimizing unnecessary side effects. For instance, patients with high PD-L1 expression may benefit more from immune checkpoint inhibitors like nivolumab and pembrolizumab, which enhance the body's immune response against cancer cells. Additionally, personalized medicine facilitates the development of combination therapies, where targeted agents are used in conjunction with immunotherapies to enhance overall treatment effectiveness. Ongoing research and clinical trials are continually expanding the repertoire of biomarkers and refining treatment algorithms to provide even more customized care. In summary, the increased focus on personalized medicine in the RCC market is leading to more precise, effective, and individualized treatment strategies. By tailoring therapies to the unique characteristics of each patient’s cancer, personalized medicine is setting new standards in RCC management and improving outcomes.
Ongoing Clinical Trials and Research:
Ongoing clinical trials and research are pivotal in advancing the renal cell carcinoma market, driving innovation and expanding treatment options for patients. One of the significant areas of focus in current research is the development of combination therapies. Researchers are investigating the synergistic effects of combining targeted therapies, such as tyrosine kinase inhibitors (TKIs), with immune checkpoint inhibitors. For example, trials exploring the combination of cabozantinib with nivolumab have shown promising results in improving overall survival rates and response rates compared to monotherapy. These studies aim to enhance treatment outcomes by leveraging the strengths of different therapeutic modalities. Another key area of research is the exploration of novel drug candidates and therapeutic targets. Clinical trials are evaluating new agents that target various pathways involved in RCC progression, such as the mTOR pathway and angiogenesis. Agents like belzutifan and avo-mab are being tested for their potential to provide additional treatment options for patients, especially those who have developed resistance to existing therapies.
Personalized medicine is also a significant focus in ongoing research. Clinical trials are investigating how genetic and molecular profiling can guide treatment decisions, allowing for more tailored and effective therapies. Biomarker-driven studies aim to identify which patients are most likely to benefit from specific drugs, thereby optimizing treatment strategies and minimizing adverse effects. Additionally, research into improving the quality of life for RCC patients is gaining traction. Trials assess supportive care interventions and strategies to manage treatment-related side effects, ensuring that patients receive comprehensive care throughout their treatment journey. In summary, ongoing clinical trials and research are crucial in shaping the future of RCC treatment. By exploring new drug combinations, novel targets, and personalized approaches, these efforts are expanding the therapeutic arsenal and offering new hope for patients battling renal cell carcinoma.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7313&method=587
Leading Companies in the Renal Cell Carcinoma Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global renal cell carcinoma market, several notable companies are actively pursuing new therapies and treatment strategies. This includes expanding clinical trials and investing in research to better understand RCC biology and improve treatment options. SillaJen Biotherapeutics and CoImmune have been investing heavily in their manufacturing capacities in recent months.
SillaJen submitted CSR to the US FDA on February 6, 2024, for REN026, a phase 1b/2a dose escalation and safety/efficacy evaluation study of Pexa-Vec in conjunction with cemiplimab in patients with metastatic or unresectable renal cell carcinoma.
Additionally, in November 2023, the FDA granted batiraxcept a fast-track designation (FTD) for patients with clear cell renal cell carcinoma who have progressed after receiving one or two previous courses of systemic therapy.
Apart from this, CoImmune published a review of its clinical development program for CMN-001, a dendritic cell-based immunotherapy electroporated with autologous tumor RNA to treat metastatic renal cell carcinoma.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7313&flag=E
Regional Analysis:
The major markets for renal cell carcinoma include the United States, Germany, France, the United Kingdom, Italy, Spain and Japan. According to projections by IMARC, the United States has the largest patient pool for renal cell carcinoma while also representing the biggest market for its treatment. This can be attributed to the growing emphasis on exploring various combination and sequential therapy approaches to improve treatment outcomes.
Moreover, the adoption of digital health technologies, including electronic health records, telemedicine, and remote monitoring tools, is improving patient management and care in RCC. These technologies enhance the ability to track patient progress, manage side effects, and ensure adherence to treatment regimens.
Apart from this, the emerging trend towards combining immunotherapies with targeted therapies, such as TKIs, is gaining momentum. Clinical trials and real-world studies are increasingly exploring combinations like nivolumab with cabozantinib, aiming to enhance efficacy and improve patient outcomes.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the renal cell carcinoma market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the renal cell carcinoma market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report offers a comprehensive analysis of current renal cell carcinoma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
IMARC Group Offer Other Reports:
Blood Pressure Monitoring Devices Market: The global blood pressure monitoring devices market size reached US$ 2.7 Billion in 2023, and projected to reach US$ 7.4 Billion by 2032, exhibiting a growth rate (CAGR) of 11.5% during the forecast period from 2024 to 2032.
Medical Device Security Market: The global medical device security market size reached US$ 9.7 Billion in 2023, and projected to reach US$ 30.6 Billion by 2032, exhibiting a growth rate (CAGR) of 13.18% during the forecast period from 2024 to 2032.
Compression Therapy Market: The global compression therapy market size reached US$ 3.9 Billion in 2023, and projected to reach US$ 6.4 Billion by 2032, exhibiting a growth rate (CAGR) of 5.48% during the forecast period from 2024 to 2032.
Glioblastoma Multiforme Treatment Market: The global glioblastoma multiforme treatment market size reached US$ 2.1 Billion in 2023, and projected to reach US$ 3.9 Billion by 2032, exhibiting a growth rate (CAGR) of 6.7% during the forecast period from 2024 to 2032.
Drug Discovery Informatics Market: The global drug discovery informatics market size reached US$ 3.3 Billion in 2023, and projected to reach US$ 7.8 Billion by 2032, exhibiting a growth rate (CAGR) of 9.7% during the forecast period from 2024 to 2032.
Ultraviolet Disinfection Equipment Market: The global ultraviolet (UV) disinfection equipment market size reached US$ 3.1 Billion in 2023, and expected to reach US$ 10.6 Billion by 2032, exhibiting a growth rate (CAGR) of 14.3% during the forecast period from 2024 to 2032.
Benign Prostatic Hyperplasia Treatment Market: The global benign prostatic hyperplasia treatment market size reached US$ 11.9 Billion in 2023, and expected to reach US$ 18.1 Billion by 2032, exhibiting a growth rate (CAGR) of 4.6% during the forecast period from 2024 to 2032.
Cannabis Infused Edible Products Market: The global cannabis infused edible products market size reached US$ 19.5 Billion in 2023, and projected to reach US$ 63.2 Billion by 2032, exhibiting a growth rate (CAGR) of 13.97% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800