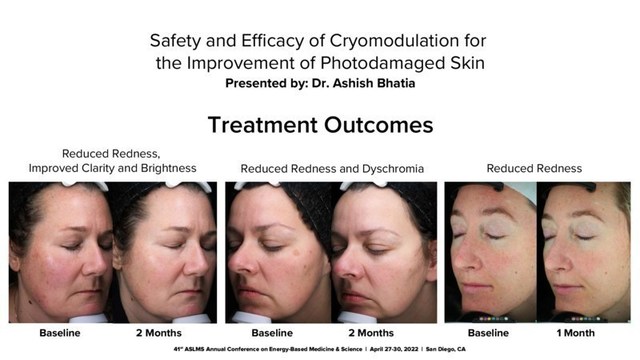
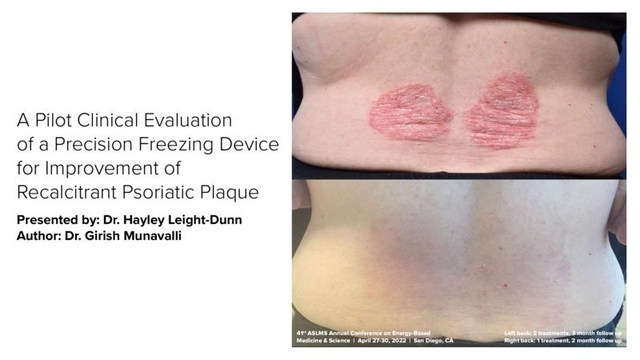
R2 Technologies Inc. today announced that data related to GLACIAL ® Rx, the first-to-market controlled cooling platform of its kind, was presented during the annual meeting of the American Society for Laser Medicine and Surgery (ASLMS) which took place from April 27-30.
|
SAN RAMON, Calif., May 3, 2022 /PRNewswire/ -- R2 Technologies Inc. ("R2"), the world leader in CryoAesthetic™ medical devices, today announced that data related to GLACIAL®Rx, the first-to-market controlled cooling platform of its kind, was presented during the annual meeting of the American Society for Laser Medicine and Surgery (ASLMS) which took place from April 27-30, in San Diego, CA. Favorable data regarding the GLACIAL®Rx device were highlighted in two oral presentations during the meeting.
"The data presented at the ASLMS meeting underscore the clinical benefits of the GLACIAL® Rx platform."
"The data presented at the ASLMS meeting underscore the clinical benefits of the GLACIAL® Rx platform, and the potential for our precision contact cooling modalities," says Tim Holt, R2's Chief Executive Officer. "These are the first academic data presented that demonstrate the benefits of controlled cooling to both improve the appearance of inflamed, photodamaged skin, and provide some symptomatic relief from painful psoriatic plaques. We will continue to invest in R&D programs and research studies that explore dermatalogical benefits of the GLACIAL® Rx platform." In addition to the data presentations, R2 hosted a Scientific Advisory Board during the ASLMS annual meeting. Advisory board attendees included the physicians who developed the GLACIAL® Rx technology including Dr. Rox Anderson, Dr. Dieter Manstein and Dr. Henry Chan. R2 recently passed a milestone with 100 commercial devices placed in the market, delivering over 15,000 treatment cycles, and will be celebrating with hashtag #Glacial100 in the coming weeks. About R2 Technologies PR CONTACT: EvolveMKD, GlacialRx@EvolveMKD.com
SOURCE R2 Technologies |