Novel antibody demonstrates highly specific and highly potent targeting of immunosuppressive γδ1 T cells from cancer patients Enriched in many intractable solid tumors and blood of cancer patients, γδ1 T cells represent important new target for cancer immunotherapy
Novel antibody demonstrates highly specific and highly potent targeting of immunosuppressive γδ1 T cells from cancer patients
Enriched in many intractable solid tumors and blood of cancer patients, γδ1 T cells represent important new target for cancer immunotherapy
BOSTON--(BUSINESS WIRE)-- PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced the presentation of a scientific poster detailing additional promising preclinical results for its LYT-210 antibody at the 2021 American Association for Cancer Research (AACR) Annual Virtual Meeting.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210412005194/en/
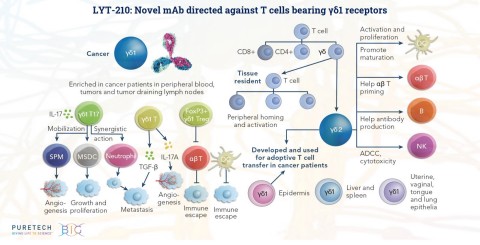
PureTech announced the presentation of a scientific poster detailing additional promising preclinical results for its LYT-210 antibody at the 2021 AACR Annual Virtual Meeting. The new research demonstrates that LYT-210 is both highly specific and highly potent, rapidly inducing cell death of immune-suppressive gamma delta-1 T cells, while sparing other T cells that play important roles in a healthy immune response. (Photo: Business Wire)
LYT-210 is a novel, fully human monoclonal antibody (mAb) directed against T cells bearing γδ1 receptors, which are known to suppress the anti-tumor immune response. The new research shared at AACR demonstrates that LYT-210 is both highly specific and highly potent, rapidly inducing cell death of immune-suppressive γδ1 T cells, while sparing other T cells that play important roles in a healthy immune response. The research was conducted in vitro using both patient blood and cancer tissue. LYT-210 has potential as either a single agent or in combination with checkpoint inhibitors and other anti-cancer treatments.
“The role of γδ1 T cells in cancer immune suppression has come into sharp focus in recent years. We now know that these cells deploy multiple immunosuppressive signals to dampen the anti-tumor response and enable the cancer to grow and spread,” said Aleksandra Filipovic, M.D. Ph.D., Head of Oncology at PureTech. “We are excited by these new data demonstrating that our LYT-210 therapeutic candidate can precisely target and swiftly deplete pathogenic γδ1 T cells. We believe that removing these culprits from the tumor microenvironment systemically may have the potential to reawaken the immune system and contribute to a strong anti-tumor response. Moreover, both we and others in the field have established that a heightened presence of pathogenic γδ1 T cells in tumor tissue and blood is correlated with more aggressive disease, poorer response to some therapies and a lower chance of survival. Given those links, we believe that the biomarker-centric approach we are developing as part of our γδ1 T cell program may have the potential to identify and select the patients who are most likely to benefit from LYT-210 in the clinic and beyond.”
γδ1 T cells are upregulated in multiple solid tumors including breast cancer, glioblastoma, melanoma and pancreatic cancer. They suppress the immune response through multiple mechanisms, including blocking effector T cells, hindering antigen-presenting dendritic cells, restricting the anti-tumoral activity of γδ2 T cells and attracting tumor-associated macrophages and myeloid-derived suppressor cells to the tumor microenvironment. Pathogenic γδ1 T cells are distinct from cytotoxic γδ T cells, which are being used for adoptive T cell transfer or therapeutic engagement with bispecific antibodies. Depleting pathogenic γδ1 T cells has the capacity to modulate both innate and adaptive immunity, and their distinct phenotypic and functional properties make them excellent potential therapeutic targets.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including intractable cancers, lymphatic and gastrointestinal diseases, central nervous system disorders and inflammatory and immunological diseases, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech’s Founded Entities, as of the date of PureTech’s most recently filed Registration Statement on Form 20-F, was comprised of 24 therapeutics and therapeutic candidates, including two that have received FDA clearance and European marketing authorization. All of the underlying programs and platforms that resulted in this pipeline of product candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company’s unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, our expectations regarding the potential therapeutic benefits of our therapeutic candidates, our expectations regarding the potential mechanism of action and related benefits expected from LYT-210 based on the preclinical results presented at AACR, and those risks and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210412005194/en/
Contacts
Investors
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
U.S. media
Stephanie Simon
+1 617 581 9333
stephanie@tenbridgecommunications.com
Source: PureTech Health plc
Smart Multimedia Gallery
PureTech announced the presentation of a scientific poster detailing additional promising preclinical results for its LYT-210 antibody at the 2021 AACR Annual Virtual Meeting. The new research demonstrates that LYT-210 is both highly specific and highly potent, rapidly inducing cell death of immune-suppressive gamma delta-1 T cells, while sparing other T cells that play important roles in a healthy immune response. (Photo: Business Wire)







