Proliferative Vitreoretinopathy Market Outlook 2024-2034:
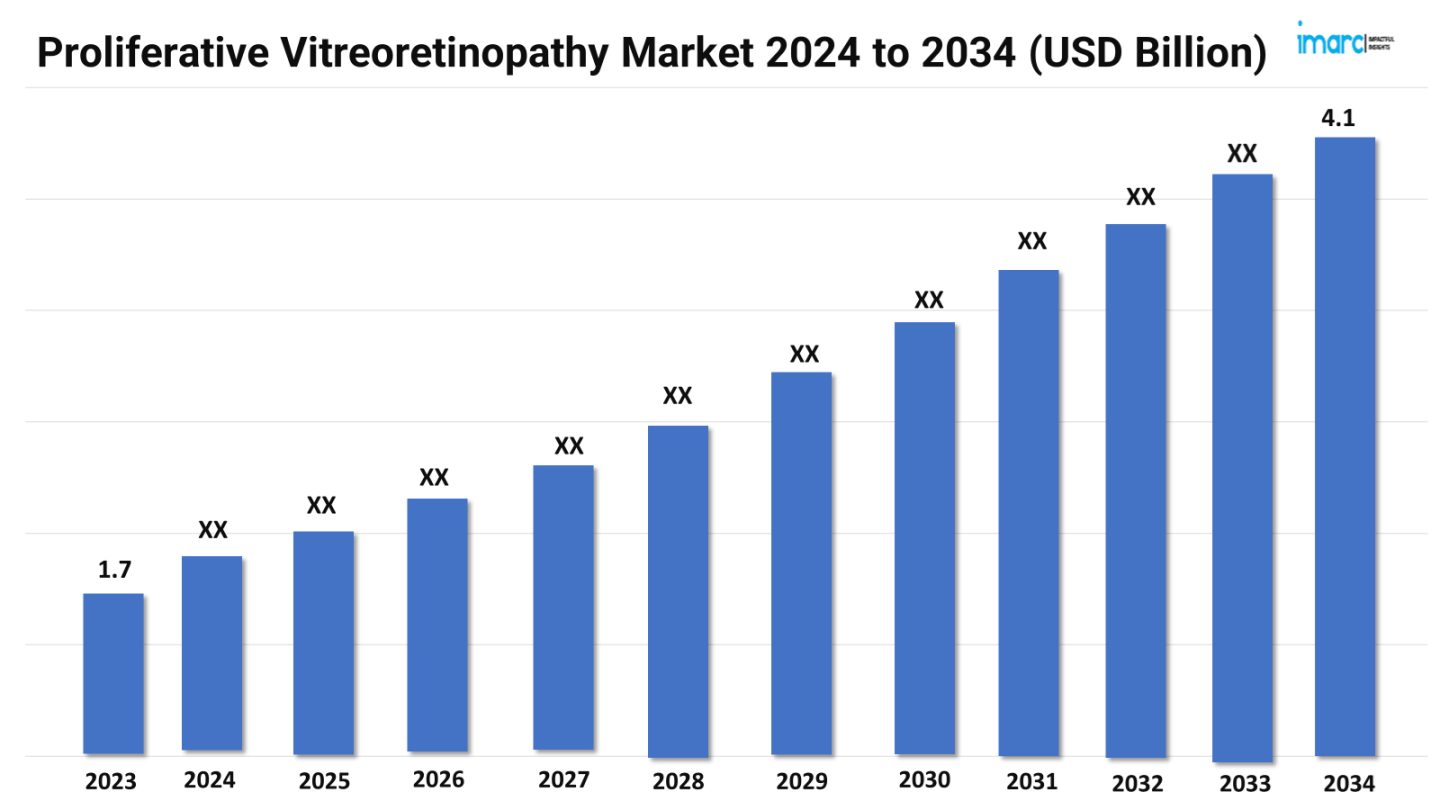
The proliferative vitreoretinopathy market size reached a value of US$ 1.7 Billion in 2023. Looking forward, the market is expected to reach US$ 4.1 Billion by 2034, exhibiting a growth rate (CAGR) of 8.45% during 2024-2034. The market is driven by novel therapeutic developments and improved diagnostic techniques. Additionally, there is growing research into gene therapy and the application of stem cell therapy to repair and regenerate retinal tissues.
Development of Novel Therapeutic Agents: Driving the Proliferative Vitreoretinopathy Market
The development of innovative treatments for Proliferative Vitreoretinopathy (PVR) is a significant emphasis area, intending to improve treatment results and lower recurrence rates after surgery. PVR is a serious consequence of retinal detachment that can cause visual loss, and existing therapies are frequently ineffective, with high rates of recurrence. Recent advances have centered on anti-inflammatory and anti-proliferative medicines that address the molecular pathways causing PVR. For example, corticosteroids and nonsteroidal anti-inflammatory medications (NSAIDs) are used to reduce the inflammatory response that causes retinal scarring and cell proliferation. Furthermore, anti-proliferative drugs such as methotrexate and 5-fluorouracil are being investigated to prevent the cellular processes that contribute to membrane development in the retina.
Request a PDF Sample Report: https://www.imarcgroup.com/proliferative-vitreoretinopathy-market/requestsample
The development of biologics and gene treatments is an excellent example of innovation in this field. Biologic medicines such as anti-vascular endothelial growth factor (anti-VEGF) therapy are being studied for their ability to prevent aberrant blood vessel development and scar tissue formation in PVR patients. Furthermore, gene therapy is a potential technique that targets particular genetic pathways implicated in the illness process. Researchers are investigating the use of viral vectors to deliver therapeutic genes capable of suppressing the biological pathways that contribute to PVR. For example, a recent study demonstrated the efficacy of a gene therapy approach that inhibits the connective tissue growth factor (CTGF), a protein implicated in fibrosis and scarring in PVR. These advancements reflect a broader trend towards precision medicine, where treatments are tailored to the molecular and genetic profile of the disease. The ongoing research and clinical trials in this area are crucial for bringing these novel therapies to market, offering hope for more effective and durable solutions for patients with PVR. As these therapies progress through the development pipeline, they hold the potential to significantly improve clinical outcomes and quality of life for those affected by this challenging condition.
Innovations in Surgical Techniques: Contributing to Market Expansion
Innovations in surgical techniques are playing a pivotal role in improving outcomes for patients with Proliferative Vitreoretinopathy (PVR). PVR, a complication of retinal detachment surgery characterized by the growth of membranes on the retina, requires advanced surgical interventions to manage effectively. Recent advancements in vitrectomy techniques have significantly enhanced the precision and success rates of these procedures. For instance, smaller gauge vitrectomy systems, such as 25-gauge and 27-gauge instruments, allow for less invasive surgeries with reduced healing times and lower risks of complications. These microincision vitrectomy surgeries (MIVS) enable surgeons to perform delicate maneuvers within the eye with greater control and minimal trauma to the ocular tissues.
One prominent example of surgical innovation is the integration of high-resolution imaging systems and intraoperative optical coherence tomography (iOCT). These technologies provide real-time, detailed visualization of the retinal structures during surgery, allowing surgeons to identify and address microscopic abnormalities that may contribute to PVR. For instance, iOCT can help surgeons ensure the complete removal of the epiretinal membranes and assess the retinal architecture's integrity immediately after membrane peeling. This real-time feedback is crucial for reducing the risk of PVR recurrence and improving postoperative visual outcomes. A study published in the journal Ophthalmology demonstrated that the use of iOCT during vitrectomy led to better anatomical and functional results in patients with complex retinal detachments complicated by PVR. These technological advancements in surgical techniques represent a significant leap forward in the management of PVR. By enhancing the precision and efficacy of surgical interventions, these innovations contribute to better patient outcomes and a higher quality of life for individuals affected by this challenging condition. As research and development continue, further improvements in surgical tools and techniques are expected to provide even more effective solutions for managing PVR.
Research in Gene and Stem Cell Therapy:
Research in gene and stem cell therapy is emerging as a groundbreaking approach in the proliferative vitreoretinopathy (PVR) market, offering promising avenues for treatment that address the condition's underlying causes rather than just managing symptoms. Gene therapy focuses on correcting genetic defects that contribute to PVR, potentially providing long-term solutions. For example, researchers are exploring the use of viral vectors to deliver therapeutic genes that can inhibit the production of proteins implicated in fibrosis and scarring. A significant development in this area involves targeting the connective tissue growth factor (CTGF), a protein associated with the fibrotic process in PVR. Stem cell therapy is another promising frontier in PVR treatment, aimed at regenerating damaged retinal tissues and restoring normal function. Stem cells have the potential to differentiate into various cell types, including those that make up the retinal structure. Research has demonstrated that stem cells can be used to repair retinal damage caused by PVR. For instance, a study conducted at the University of California, San Francisco, involved injecting stem cells into the vitreous cavity of the eye, where they successfully integrated into the damaged retina and promoted tissue regeneration. This approach not only helps repair the retinal structure but also restores visual function, offering a potential cure rather than a temporary fix.
These advancements in gene and stem cell therapies represent a paradigm shift in the management of PVR, moving from symptomatic treatment to addressing the root causes of the disease. The ongoing clinical trials and research efforts are crucial for validating these therapies' safety and efficacy, paving the way for their integration into standard clinical practice. As these innovative treatments continue to develop, they hold the promise of significantly improving outcomes for patients with PVR, reducing recurrence rates, and enhancing the quality of life for those affected by this challenging condition.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8017&method=587
Leading Companies in the Proliferative Vitreoretinopathy Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global proliferative vitreoretinopathy market, several key companies are leading the way in the research and development of new therapies and treatments. Some of the major players include Aldeyra Therapeutics and Tetra Bio-Pharma. These companies focus on various innovative approaches, including surgical techniques, gene therapy, and stem cell research to address the challenges posed by PVR.
Aldeyra is a prominent player in the PVR market, known for its development of ADX-2191, an intravitreal formulation of methotrexate. This drug has been granted orphan drug designation for the prevention of PVR. The clinical activity of ADX-2191 is believed to down-regulate aberrant retinal cell proliferation and reduce retinal scarring, which are key factors in PVR development.
Moreover, Tetra Bio-Pharma's anticipated launch of REDUVO™ and REDUVO™ Adversa® demonstrates its expanding capabilities in cannabinoid-based pharmaceuticals. While these products are focused on addressing CINV, the company's work on cannabinoid therapies has broader implications, including potential treatments for other conditions such as proliferative vitreoretinopathy (PVR). Tetra Bio-Pharma's synthetic cannabinoid PPP003 (HU308) has been granted orphan-drug status by the FDA for the prevention of PVR, indicating the company's strategic move to leverage its expertise in cannabinoids to address various unmet medical needs.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8017&flag=E
Regional Analysis:
The major markets for proliferative vitreoretinopathy include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for proliferative vitreoretinopathy while also representing the biggest market for its treatment. This can be attributed to advancements in medical research, innovative therapeutic approaches, and an increasing focus on improving surgical techniques.
Moreover, innovations in vitrectomy procedures and the use of adjunctive pharmacological agents are enhancing the precision and success rates of surgeries to treat PVR. The development of smaller gauge vitrectomy systems and improved intraoperative imaging technologies, such as intraoperative optical coherence tomography (iOCT), are pivotal in reducing recurrence rates and improving patient outcomes.
Besides this, there is a growing focus on developing novel therapeutic agents to manage and prevent PVR. These include anti-inflammatory and anti-proliferative drugs that target the molecular mechanisms underlying the disease. For instance, Aldeyra Therapeutics is advancing ADX-2191, an intravitreal formulation of methotrexate, which has shown promise in reducing retinal scarring and cell proliferation associated with PVR.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the proliferative vitreoretinopathy market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the proliferative vitreoretinopathy market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current proliferative vitreoretinopathy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/proliferative-vitreoretinopathy-market
IMARC Group Offer Other Reports:
Neuroectodermal Tumors Market: The 7 major neuroectodermal tumors market is expected to exhibit a CAGR of 1.07% during the forecast period from 2024 to 2034.
Bronchiectasis Market: The 7 major bronchiectasis market reached a value of US$ 438.5 Million in 2023, and projected the 7MM to reach US$ 731.5 Million by 2034, exhibiting a growth rate (CAGR) of 4.76% during the forecast period from 2024 to 2034.
Respiratory Syncytial Virus Market: The 7 major respiratory syncytial virus market reached a value of US$ 1.3 Billion in 2023, and projected the 7MM to reach US$ 3.8 Billion by 2034, exhibiting a growth rate (CAGR) of 10.01% during the forecast period from 2024 to 2034.
Cervical Cancer Market: The 7 major cervical cancer market reached a value of US$ 405.6 Million in 2023, and projected the 7MM to reach US$ 583.4 Million by 2034, exhibiting a growth rate (CAGR) of 3.36% during the forecast period from 2024 to 2034.
Primary Biliary Cholangitis Market: The 7 major primary biliary cholangitis market reached a value of US$ 510 Million in 2023, and projected the 7MM to reach US$ 1,200 Million by 2034, exhibiting a growth rate (CAGR) of 8.06% during the forecast period from 2024 to 2034.
Stress Urinary Incontinence Market: The 7 major stress urinary incontinence market is expected to exhibit a CAGR of 6.14% during the forecast period from 2024 to 2034.
Palmar Hyperhidrosis Market: The 7 major palmar hyperhidrosis market reached a value of US$ 231.1 Million in 2023, and projected the 7MM to reach US$ 352.6 Million by 2034, exhibiting a growth rate (CAGR) of 3.92% during the forecast period from 2024-2034.
T-Cell Non-Hodgkin Lymphoma Market: The 7 major T-cell non-hodgkin lymphoma market is expected to exhibit a CAGR of 5.55% during the forecast period from 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The proliferative vitreoretinopathy market size reached a value of US$ 1.7 Billion in 2023. Looking forward, the market is expected to reach US$ 4.1 Billion by 2034, exhibiting a growth rate (CAGR) of 8.45% during 2024-2034. The market is driven by novel therapeutic developments and improved diagnostic techniques. Additionally, there is growing research into gene therapy and the application of stem cell therapy to repair and regenerate retinal tissues.
Development of Novel Therapeutic Agents: Driving the Proliferative Vitreoretinopathy Market
The development of innovative treatments for Proliferative Vitreoretinopathy (PVR) is a significant emphasis area, intending to improve treatment results and lower recurrence rates after surgery. PVR is a serious consequence of retinal detachment that can cause visual loss, and existing therapies are frequently ineffective, with high rates of recurrence. Recent advances have centered on anti-inflammatory and anti-proliferative medicines that address the molecular pathways causing PVR. For example, corticosteroids and nonsteroidal anti-inflammatory medications (NSAIDs) are used to reduce the inflammatory response that causes retinal scarring and cell proliferation. Furthermore, anti-proliferative drugs such as methotrexate and 5-fluorouracil are being investigated to prevent the cellular processes that contribute to membrane development in the retina.
Request a PDF Sample Report: https://www.imarcgroup.com/proliferative-vitreoretinopathy-market/requestsample
The development of biologics and gene treatments is an excellent example of innovation in this field. Biologic medicines such as anti-vascular endothelial growth factor (anti-VEGF) therapy are being studied for their ability to prevent aberrant blood vessel development and scar tissue formation in PVR patients. Furthermore, gene therapy is a potential technique that targets particular genetic pathways implicated in the illness process. Researchers are investigating the use of viral vectors to deliver therapeutic genes capable of suppressing the biological pathways that contribute to PVR. For example, a recent study demonstrated the efficacy of a gene therapy approach that inhibits the connective tissue growth factor (CTGF), a protein implicated in fibrosis and scarring in PVR. These advancements reflect a broader trend towards precision medicine, where treatments are tailored to the molecular and genetic profile of the disease. The ongoing research and clinical trials in this area are crucial for bringing these novel therapies to market, offering hope for more effective and durable solutions for patients with PVR. As these therapies progress through the development pipeline, they hold the potential to significantly improve clinical outcomes and quality of life for those affected by this challenging condition.
Innovations in Surgical Techniques: Contributing to Market Expansion
Innovations in surgical techniques are playing a pivotal role in improving outcomes for patients with Proliferative Vitreoretinopathy (PVR). PVR, a complication of retinal detachment surgery characterized by the growth of membranes on the retina, requires advanced surgical interventions to manage effectively. Recent advancements in vitrectomy techniques have significantly enhanced the precision and success rates of these procedures. For instance, smaller gauge vitrectomy systems, such as 25-gauge and 27-gauge instruments, allow for less invasive surgeries with reduced healing times and lower risks of complications. These microincision vitrectomy surgeries (MIVS) enable surgeons to perform delicate maneuvers within the eye with greater control and minimal trauma to the ocular tissues.
One prominent example of surgical innovation is the integration of high-resolution imaging systems and intraoperative optical coherence tomography (iOCT). These technologies provide real-time, detailed visualization of the retinal structures during surgery, allowing surgeons to identify and address microscopic abnormalities that may contribute to PVR. For instance, iOCT can help surgeons ensure the complete removal of the epiretinal membranes and assess the retinal architecture's integrity immediately after membrane peeling. This real-time feedback is crucial for reducing the risk of PVR recurrence and improving postoperative visual outcomes. A study published in the journal Ophthalmology demonstrated that the use of iOCT during vitrectomy led to better anatomical and functional results in patients with complex retinal detachments complicated by PVR. These technological advancements in surgical techniques represent a significant leap forward in the management of PVR. By enhancing the precision and efficacy of surgical interventions, these innovations contribute to better patient outcomes and a higher quality of life for individuals affected by this challenging condition. As research and development continue, further improvements in surgical tools and techniques are expected to provide even more effective solutions for managing PVR.
Research in Gene and Stem Cell Therapy:
Research in gene and stem cell therapy is emerging as a groundbreaking approach in the proliferative vitreoretinopathy (PVR) market, offering promising avenues for treatment that address the condition's underlying causes rather than just managing symptoms. Gene therapy focuses on correcting genetic defects that contribute to PVR, potentially providing long-term solutions. For example, researchers are exploring the use of viral vectors to deliver therapeutic genes that can inhibit the production of proteins implicated in fibrosis and scarring. A significant development in this area involves targeting the connective tissue growth factor (CTGF), a protein associated with the fibrotic process in PVR. Stem cell therapy is another promising frontier in PVR treatment, aimed at regenerating damaged retinal tissues and restoring normal function. Stem cells have the potential to differentiate into various cell types, including those that make up the retinal structure. Research has demonstrated that stem cells can be used to repair retinal damage caused by PVR. For instance, a study conducted at the University of California, San Francisco, involved injecting stem cells into the vitreous cavity of the eye, where they successfully integrated into the damaged retina and promoted tissue regeneration. This approach not only helps repair the retinal structure but also restores visual function, offering a potential cure rather than a temporary fix.
These advancements in gene and stem cell therapies represent a paradigm shift in the management of PVR, moving from symptomatic treatment to addressing the root causes of the disease. The ongoing clinical trials and research efforts are crucial for validating these therapies' safety and efficacy, paving the way for their integration into standard clinical practice. As these innovative treatments continue to develop, they hold the promise of significantly improving outcomes for patients with PVR, reducing recurrence rates, and enhancing the quality of life for those affected by this challenging condition.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8017&method=587
Leading Companies in the Proliferative Vitreoretinopathy Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global proliferative vitreoretinopathy market, several key companies are leading the way in the research and development of new therapies and treatments. Some of the major players include Aldeyra Therapeutics and Tetra Bio-Pharma. These companies focus on various innovative approaches, including surgical techniques, gene therapy, and stem cell research to address the challenges posed by PVR.
Aldeyra is a prominent player in the PVR market, known for its development of ADX-2191, an intravitreal formulation of methotrexate. This drug has been granted orphan drug designation for the prevention of PVR. The clinical activity of ADX-2191 is believed to down-regulate aberrant retinal cell proliferation and reduce retinal scarring, which are key factors in PVR development.
Moreover, Tetra Bio-Pharma's anticipated launch of REDUVO™ and REDUVO™ Adversa® demonstrates its expanding capabilities in cannabinoid-based pharmaceuticals. While these products are focused on addressing CINV, the company's work on cannabinoid therapies has broader implications, including potential treatments for other conditions such as proliferative vitreoretinopathy (PVR). Tetra Bio-Pharma's synthetic cannabinoid PPP003 (HU308) has been granted orphan-drug status by the FDA for the prevention of PVR, indicating the company's strategic move to leverage its expertise in cannabinoids to address various unmet medical needs.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8017&flag=E
Regional Analysis:
The major markets for proliferative vitreoretinopathy include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for proliferative vitreoretinopathy while also representing the biggest market for its treatment. This can be attributed to advancements in medical research, innovative therapeutic approaches, and an increasing focus on improving surgical techniques.
Moreover, innovations in vitrectomy procedures and the use of adjunctive pharmacological agents are enhancing the precision and success rates of surgeries to treat PVR. The development of smaller gauge vitrectomy systems and improved intraoperative imaging technologies, such as intraoperative optical coherence tomography (iOCT), are pivotal in reducing recurrence rates and improving patient outcomes.
Besides this, there is a growing focus on developing novel therapeutic agents to manage and prevent PVR. These include anti-inflammatory and anti-proliferative drugs that target the molecular mechanisms underlying the disease. For instance, Aldeyra Therapeutics is advancing ADX-2191, an intravitreal formulation of methotrexate, which has shown promise in reducing retinal scarring and cell proliferation associated with PVR.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the proliferative vitreoretinopathy market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the proliferative vitreoretinopathy market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current proliferative vitreoretinopathy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/proliferative-vitreoretinopathy-market
IMARC Group Offer Other Reports:
Neuroectodermal Tumors Market: The 7 major neuroectodermal tumors market is expected to exhibit a CAGR of 1.07% during the forecast period from 2024 to 2034.
Bronchiectasis Market: The 7 major bronchiectasis market reached a value of US$ 438.5 Million in 2023, and projected the 7MM to reach US$ 731.5 Million by 2034, exhibiting a growth rate (CAGR) of 4.76% during the forecast period from 2024 to 2034.
Respiratory Syncytial Virus Market: The 7 major respiratory syncytial virus market reached a value of US$ 1.3 Billion in 2023, and projected the 7MM to reach US$ 3.8 Billion by 2034, exhibiting a growth rate (CAGR) of 10.01% during the forecast period from 2024 to 2034.
Cervical Cancer Market: The 7 major cervical cancer market reached a value of US$ 405.6 Million in 2023, and projected the 7MM to reach US$ 583.4 Million by 2034, exhibiting a growth rate (CAGR) of 3.36% during the forecast period from 2024 to 2034.
Primary Biliary Cholangitis Market: The 7 major primary biliary cholangitis market reached a value of US$ 510 Million in 2023, and projected the 7MM to reach US$ 1,200 Million by 2034, exhibiting a growth rate (CAGR) of 8.06% during the forecast period from 2024 to 2034.
Stress Urinary Incontinence Market: The 7 major stress urinary incontinence market is expected to exhibit a CAGR of 6.14% during the forecast period from 2024 to 2034.
Palmar Hyperhidrosis Market: The 7 major palmar hyperhidrosis market reached a value of US$ 231.1 Million in 2023, and projected the 7MM to reach US$ 352.6 Million by 2034, exhibiting a growth rate (CAGR) of 3.92% during the forecast period from 2024-2034.
T-Cell Non-Hodgkin Lymphoma Market: The 7 major T-cell non-hodgkin lymphoma market is expected to exhibit a CAGR of 5.55% during the forecast period from 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800