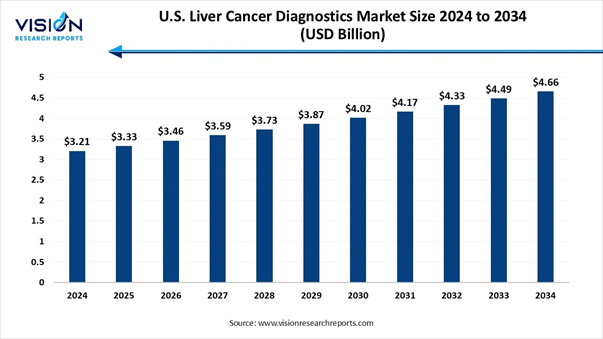
The U.S. liver cancer diagnostics market size reached at USD 3.21 billion in 2024 and is expected to grow from USD 3.33 billion in 2025 to reach around USD 4.66 billion by 2034, expanding at a CAGR of 3.8% from 2025 to 2034, a study published by Vision Research Reports.
The growth is due to U.S. liver cancer diagnostics, which is being driven by the developing worldwide prevalence of liver cancer, as well as the complicated demand for early detection and major advancements in diagnostic technologies.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Preview the Report Before You Buy – Get Sample Pages 👉 https://www.visionresearchreports.com/report/sample/41376
What is the Liver Cancer Diagnostics Market?
Liver cancer diagnosis is the procedure of classifying malignant development in the liver, which is most commonly known as hepatocellular carcinoma (HCC). The diagnostic procedure includes an integration of a physical examination, blood tests, and medical history, and lastly, advanced imaging.
The liver is the biggest internal organ and has many necessary functions, including metabolism, storage, and detoxification of different nutrients. Various types of cells in the liver perform these functions. These cells have an agreed division design depending on the information present in the genes. At this time, these cells may precede their crafted functions and begin classifying abnormally because of mutations that lead to the creation of a cancerous tumor or mass.
With instance to this,
• The American Cancer Society has calculated that for initial liver cancer and intrahepatic bile duct cancer in the United States for the year 2025, there will be about 42,240 new cases (28,220 and 14,00 in women), which can be treated. (Source: https://www.cancer.org)
U.S. Liver Cancer Diagnostics Market Key Highlights:
• By test type, the laboratory tests segment accounted for the largest market share of 40% in 2024.
• By end use, the hospitals and diagnostic laboratories segment held the leading share of 49.32% in 2024.
• By end use, the pharmaceutical and Contract Research Organization (CRO) laboratories segment is projected to register the fastest growth, expanding at a CAGR of 8.09% from 2025 to 2034.
Latest Trends in the U.S. Liver Cancer Diagnostics Market
• Liquid biopsy: This developing method tracks biomarkers like shifting tumour DNA (ctDNA) from blood or other bodily fluids, which allows real-time monitoring of the tumor’s genetic makeup and feedback to therapy. In the main development, the FDA has granted Breakthrough Device Designation to the EvoLiver in early 2025 for tracking hepatocellular carcinoma in high-risk patients with cirrhosis.
• AI-powered image analysis: Algorithms are heavily mixed into diagnostic tools to track medical images like MRI scans and CT scans. These systems assist radiologists in detecting small lesions and abnormalities that may be missed by human eyes, thereby improving diagnostic accuracy.
• Digital pathology: The usage of high-resolution digital images of tissue samples develops diagnostic workflows by allowing remote access for partnerships among the experts and enhancing smoothness.
• Biopsy for specific cases: Biopsy is heavily booked for cases in which imaging is inconclusive, specifically for smaller tumors or for diagnosis in patients without giving importance to cirrhosis. For such cases, biopsy serves complicated tissue for genetic analysis, which is necessary for guiding personalized treatment and immunotherapy.
• Enhanced biomarker panels: While regular markers like alpha-fetoprotein (AFP) have restricted sensitivity, new tests are integrating multiple biomarkers for more accurate diagnosis and screening, too. Developing biomarkers include AFP-L3, Des-gamma carboxyprothrombin and Glypican that can be developed in HCC patients.
U.S. Liver Cancer Diagnostics Government Initiatives
• American Liver Foundation (ALF), the U.S Department of Veterans Affairs, and the Veterans Health Administration are delighted to reveal the collaboration in order to assist Veterans who are either at risk or living with liver disease. (Source: https://liverfoundation.org)
Discover the Full Market Insights 👉
https://www.visionresearchreports.com/us-liver-cancer-diagnostics-market/41376
U.S. Liver Cancer Diagnostics Market Dynamics
Opportunity
What is the Major Market Opportunity for the U.S. Liver Cancer Diagnostics Market?
Researchers have currently conducted a systematic review of AI in treating HCC by using CT imaging. The searches displayed AI performed perfectly enough to be mixed into routine radiology practice. Also, in the year 2025, scientists found that integrating finotonclimb with SCT510 has stretched survival in high-level HCC patients as compared to the long-standing standard diagnosis called sorafenib. Out of the 1,580 actual clinical trials that test CAR-T cell therapies, around 15% focus on gallbladder, liver, and pancreatic cancers. These trials have featured in rising interest in terms of CAR-T as a capable option for liver cancers. Also, AI assists in lowering the analysis errors and accelerates communication by helping radiologists to deliver through large volumes of work.
Limitation
What is the Limitation for the Growth of U.S. Liver Cancer Diagnostics Market?
The challenges associated with liver cancer are that present therapies, which include surgery, targeted treatments, and liver transplants, can develop results for some patients, but for several, smooth long-term solutions remain out of reach. The risk factors like chronic hepatitis B and C infections, alcohol-linked liver damage contribute to the rising burden of this disease and fatty liver disease too. Though there is growth in research, liver cancer remains a major challenge that features the demand for perfect screening, novel treatment strategies, and earlier detection too.
Top Technological Advancements in the U.S. Liver Cancer Diagnostics Market:
• A high-precision procedure for the liver-lung shunt fraction (LSF) quantification by using the 3D SPECT/CT images relies on CNNs and non-rigid registration that is utilized to avoid radiation pneumonitis in selective internal radiation therapy.
• A multimodal deep neural link for the multiclass malignant liver diagnosis. In parallel with the portal venous CT scans, pathology data are used to prognosticate initial liver cancer variants and metastasis.
• Deep learning, depending on the algorithms, will shift learning, enabling the making of fully automated and precise expectation models for gaining liver fibrosis stages.
U.S. Liver Cancer Diagnostics Market Report Coverage
|
Report Attribute |
Key Statistic |
|
Market Size in 2025 |
USD 3.33 Billion |
|
Market Size in 2026 |
USD 3.46 Billion |
|
Market Size in 2030 |
USD 4.02 Billion |
|
Market Size in 2032 |
USD 4.33 Billion |
|
Market Size by 2034 |
USD 4.66 Billion |
|
Growth rate from 2025 to 2034 |
CAGR of 3.8% |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Segments Covered |
By Test Type, By End-use |
|
Companies Covered |
Abbott Laboratories, Guardant Health, Thermo Fisher Scientific Inc., Illumina Inc., F. Hoffmann-La Roche Ltd., Qiagen N.V., Siemens Healthineers, Becton, Dickinson & Company, Epigenomics AG, Koninklijke Philips N.V., and FUJIFILM Healthcare Americas Corporation. |
Need a Tailored Version of the Report? | Get Customization Options Here: https://www.visionresearchreports.com/report/customization/41376
U.S. Liver Cancer Diagnostics Market Segmentation Analysis
Test Type Analysis
The laboratory tests have dominated the U.S. liver cancer diagnostics market in 2024 as Alpha-fetoprotein blood (AFP) is a protein that at some period can be searched at higher levels in the blood of people with liver disease, liver cancer (or some other cancers), and other conditions too. If the AFP levels are very high in someone with a liver tumor, it can be a sign that liver cancer is currently present. But several people with early liver cancer have regular levels of AFP, with high AFP levels, which are not very helpful in checking if a liver mass might be cancer.
Laboratory tests for liver cancer include tracking of blood for the tumor markers and checking liver function, but they are most effective when integrated with imaging scans and, in some cases, a biopsy. This strategy is compulsory due to a single blood test that cannot definitely diagnose liver cancer.
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer, which is heavily characterized by a complicated ecosystem with marked temporal and spatial heterogeneity as well as varied sensitivities to diagnosis. Current updates in the management of HCC, specifically the revelation of many immune checkpoints, are inhibitor-based regimens that keep pressing demands or more personalized imaging-aided prognostic decision-making for particular treatment selection beyond the actual “one-size-fits-all” tumor burden measurement.
Once the liver cancer is diagnosed, the doctor will perform an operation to check the extent of the cancer. Staging tests assist in checking the location and size of cancer and whether it has spread or not. Imaging test utilised to stage liver cancer, counts MRIs and CTs, and the bone scans too. There are various procedures for staging liver cancer. For instance, one method uses the Roman numerals I with the assistance of IV, and another uses letters A through D.
End-Use Analysis
The hospitals and diagnostic laboratories have dominated the market in 2024 as liver cancer is checked by utilising an integration of methods. The diagnostic method generally begins with a complete check of the patient’s medical history, which is followed by a physical examination. At that time, the doctor examines for any signs of liver cancer, such as a large spleen or liver, or ascites or jaundice. After the beginning, the doctor utilises several diagnostic tests and procedures, such as Imaging Tests. Imaging procedures are important for checking liver tumours and determining the correct size, extent, and location.
The pharmaceutical and Contract Research Organization (CRO) segment is predicted to rise at the fastest rate. Contract research organization (CRO) and the pharmaceutical industry do not directly treat liver cancer, so they make and check diagnostic tools that are used by healthcare providers, and conduct clinical trials that prove their effectiveness. Patients are diagnosed by the medical professionals by using the established clinical methods, imaging, and, if necessary, biopsy.
Pharmaceutical organisations and industry high-level contrast agents utilised in imaging tests, like magnetic, Hepatocyte-specific contrast agents such as Gd-EOB-DTPA, mainly improve the detection rate of early-stage liver cancer by highlighting liver lesions.
Browse More Related Insights:
• Europe Bladder Cancer Diagnostics Market: https://www.visionresearchreports.com/europe-bladder-cancer-diagnostics-market/41627
• Blood Cancer Diagnostics Market: https://www.visionresearchreports.com/blood-cancer-diagnostics-market/41519
• Skin Cancer Diagnostics Market: https://www.visionresearchreports.com/skin-cancer-diagnostic-market/41462
• Lung Cancer Liquid Biopsy Market: https://www.visionresearchreports.com/lung-cancer-liquid-biopsy-market/41333
• Cancer Diagnostics Market: https://www.visionresearchreports.com/cancer-diagnostics-market/41289
• Prostate Cancer Diagnostics Market: https://www.visionresearchreports.com/prostate-cancer-diagnostics-market/41101
• Liver Cancer Drug Market: https://www.visionresearchreports.com/liver-cancer-drug-market/40952
Recent Developments in the U.S. Liver Cancer Diagnostics Market
• In November 2025, Exact Sciences Corporation is a top provider of cancer screening and diagnostic tests. Currently, it has revealed important clinical validation outcomes from the ALTUS Study. (Source: https://www.exactsciences.com)
• In April 2025, the FDA approved the innovative EvoLiver test device for the supervision of hepatocellular carcinoma (HCC) in patients who have high-risk cirrhosis, as per the news released from the drug’s developer, Musla Bio. (Source: https://www.cancernetwork.com)
• In May 2025, Elecsys PRO-C3 was utilised with the ADAPT formula (age, diabetes status, and the PRO-C3 platelets), which has estimated liver fibrosis severity, a disease that is responsible for appropriately one in every 25 deaths globally. (Source: https://www.roche.com)
• In August 2025, the cancer diagnostic organization BiBBInstruments AB (“BiBB or the “Company”) revealed that Henry Ford St.John Hospital and Medical Center had tracked the primary U.S case range with the EndoDrill GI, which counts endoscopic ultrasound-guided liver biopsies. (Source: https://firstwordhealthtech.com)
• In July 2025, Olympus Corporation, which is a worldwide medical technology company that is committed to creating healthier, fulfilling, and safer, has disclosed the U.S launch of the next-generation EU-ME3 Ultrasound Processor, which can mix endoscopic and endobronchial ultrasound on a single workstation. (Source: https://www.prnewswire.com)
Top Companies in the U.S. Liver Cancer Diagnostics Market:
• Guardant Health
• Abbott Laboratories
• Thermo Fisher Scientific, Inc
• F.Hoffmann-La Roche Ltd
• Qiagen N.V
• Becton, Dickinson and Company
• Koninklijke Philips N.V
• FUJIFILM Healthcare Americas Corporation
U.S. Liver Cancer Diagnostics Market Segmentation
By Test Type
• Laboratory Tests
• Biomarkers
• Oncofetal and Glycoprotein Antigens
• Enzymes and Isoenzymes
• Growth Factors and Receptors
• Molecular Markers
• Pathological Biomarkers
• Blood Tests
• Imaging
• Endoscopy
• Biopsy
• Others
By End-use
• Hospitals & Diagnostic Laboratories
• Academic & Research Institutes
• Pharmaceutical & CRO Laboratories
Instant Delivery Available | Purchase This Exclusive Research Report Now: https://www.visionresearchreports.com/report/checkout/41376
You can place an order or ask any questions, please feel free to contact at: sales@visionresearchreports.com
About Us
Vision Research Reports is a premier service provider offering strategic market insights and solutions that go beyond traditional surveys. We specialize in actionable market research, delivering in-depth qualitative insights and strategies to global industry leaders and executives, helping them navigate future uncertainties. Our offerings include consulting services, syndicated market studies, and bespoke research reports.
We are committed to excellence in qualitative market research, fostering a team of experts with deep industry knowledge. Our goal is to help clients understand both current and future market trends, empowering them to expand their portfolios and achieve their business objectives with the right guidance.
Web: https://www.visionresearchreports.com
Our Trusted Data Partners
Precedence Research | Statifacts | Nova One Advisor
For Latest Update Follow Us: LinkedIn




