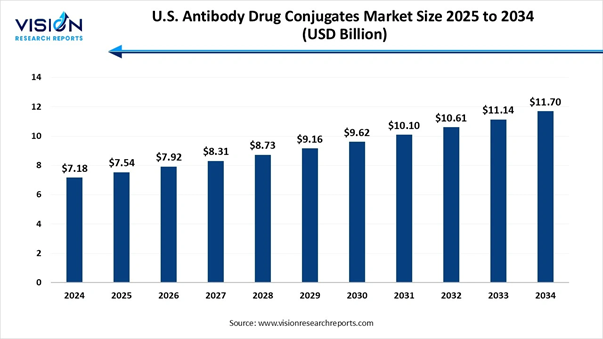
The U.S. antibody drug conjugates market size stood at USD 7.18 billion in 2024 and is predicted to increase from USD 7.54 billion in 2025 to reach approximately USD 11.70 billion by 2034, expanding at a CAGR of 5% over the forecast period, a study published by Vision Research Reports.
The growth is due to the reason that ADCs integrate the particularness of monoclonal antibodies with the capability of cytotoxic drugs that serves the drug directly to cancer cells while lowering down the damage to the health problems.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Preview the Report Before You Buy – Get Sample Pages 👉 https://www.visionresearchreports.com/report/sample/41773
What is the U.S Antibody Drug Conjugates Market?
Antibody -drug-conjugates (ADC) mixes targeted therapy and chemotherapy in order to diagnose particular blood cancers and cancerous tumors. Oncologists (cancer specialists) can use an ADC whenever cancers come back early (recur), as other diagnoses aren't so smooth (refractory cancer) or the cancers that are spread (metastasize).
This diagnosis works by focusing on particular cancerous cells and serving small doses of very rigid chemotherapy into the cells. The chemotherapy drugs destroy and damage these cancerous cells but do not harm proximate healthy cells.
The most main benefit is how ADCs can serve with chemotherapy drugs directly to the interior of cancerous cells that do not damage nearby healthy cells.
Top drugs which are used to diagnose several types of cancer like
• Brentuximab vedotin: This drug was initially ADc in order to receive an FDA approval. Its diagnosis for the repat Hodgkin lymphoma and anaplastic big cell lymphoma is among the other blood cancers list.
• Sacitatuzumab govitecan : This drug is a diagnosis for triple-negative breast cancer as when the surgery is not an option, or the cancer is spreading. It’s also being diagnosed for metastatic urothelial cancer.
Antibody Drug Conjugates Approved by the U.S Antibody Drug Conjugates Market
|
Company |
Drug |
Indication |
Year of first FDA Approval |
|
AbbVie |
Emrelis (telisotuzumab vedotin) |
Locally of high -level or metastatic non-squamous non-small cell lung cancer |
2025 |
|
AbbVie* |
Elahere (mirvetuximab soravtansine-gyxn) |
Ovarian cancer |
2022 |
|
AstraZeneca |
Lumoxiti (moxetumomab pasudotox) |
Hairy cell leukemia |
2018 |
|
ADC Therapeutics |
Zynlonta (loncastuximab tesirine-lpyl) |
Large B-cell lymphoma |
2021 |
|
AstraZeneca/Daiichi Sankyo |
Enhertu (trastuzumab deruxtecan) |
HER2-positive breast cancer |
2019 |
|
AstraZeneca/Daiichi Sankyo |
Datroway (datapotamab deruxtecean ) |
It is metastatic HR+ ,her-2 Breast cancer |
2025 |
|
Glied Sciences |
Tradelvery ( sactuzumab gavitecan |
Triple-negative breast cancer |
2020 |
|
GSK |
Blenrep (belantamab mafodotin-blmf) |
Multiple myeloma |
2020 |
|
Pfizer/ |
Adcetris (brentuximab vedotin) |
Relapsed HL and relapsed sALCL |
2011 |
|
Pfizer |
Mylotarg (gemtuzumab ozogamicin) |
Relapsed acute myelogenous leukemia |
2000, 2017 |
|
Pfizer |
Besponsa (inotuzumab ozogamicin |
CD22-positive B-cell precursor acute lymphoblastic leukemia |
2018 |
|
Pfizer/ |
Adcetris (brentuximab vedotin |
Relapsed HL and relapsed sALCL |
2011 |
|
Pfizer/ |
Padcev (enfortumab vedotin) |
Urothelial cancer |
2019 |
|
Roche |
Kadcyla (trastuzumab emtansine |
HER2-positive metastatic breast cancer |
2013 |
|
Roche |
Polivy (polatuzumab vedotin-piiq |
Diffuse large B-cell lymphoma |
2019 |
Latest
Trends in the U.S. Antibody Drug Conjugates Market •
Novel and promising designs of ADC -Bispecific ADCs: The initial source of ADC
toxicity is the particular combining of the antibodies to antigens that
develops the specificity and selectivity of ADACs which can greatly avoid
toxicity and develop their potential use cases. •
Sino Biological's efforts are on the latest trends of ADC development: Sino Biological stands
confidently at the frontline of growth in ADC research and with an overall
suite of products and services. The organization serves outstanding ADc
development solutions that substitute the path from early discovery to clinical
study to fully assisting clients during the ADC development. •
ADCs beyond oncology:
Although ADCs have mostly been created for cancer therapy, their working of
action is also usable for other non-oncological diseases, like muscular
conditions, atherosclerosis and infectious conditions, particularly for the
autoimmune conditions. •
ADC development linker:
Linkers are important in antibody -drug conjugates (ADC) as they link the
antibody and cytotoxic payload that plays an important role in the payload
release, stability and pharmacokinetics of ADC. A perfect linker should stay
stable in the circulatory machine to prevent premature drug release and lower
down off-target toxicity while allowing specific payload release in the target
tissue. •
Dual payload:
The dual-payload strategy concentrates on integration multiple working of
action to develop the therapeutic results. By integrating two different drugs,
dual-payload ADcs goal is to reduce the likeness of drug opposition, improve
anti-tumor and mitigate toxic side effects. •
ADC development -payload: Payload,
which is a cytotoxic agent responsible for killing the cancer cells, which are
complicated in encouraging the anti-tumor efficacy and capable adverse effects
of ADC. A perfect payload should release high cytotoxicity (IC50 Values) that
has low immunogenicity, modifiable functional groups and the stability in
circulation. Discover
the Full Market Insights 👉 https://www.visionresearchreports.com/us-antibody-drug-conjugates-market/41773 U.S.
Antibody Drug Conjugates Market Dynamics Opportunity How
Does Next-gen Acts as a Potential for the U.S. Antibody Drug Conjugates Market? Antibody
drug conjugates have shown a developing future with growth in site-particular
ADC conjugation services that develops smoothness and accuracy. There are
rising next-generation payloads and linker technologies that are under
development in order to develop the therapeutic index of ADCs. Hence, the
growth of ADC technology beyond oncology into sectors like neurology and immunotherapy is
gaining attention. This classification opens the latest channel for diagnosis
and serves opportunities for innovation. There are increasing collaborations
between Contract Development and Manufacturing Organizations and pharmaceutical companies too which are
predicted to streamline big-scale manufacturing that make sure these effective
therapies have become successful. With
instance to this, •
Lisata Therapeutics, is a cynical -stage pharmaceutical organisation that has
developed cutting-edge therapies for the diagnosis of high level solid tumors
and other serious diseases and the Catalent.Inc, which is a top in allowing the
supply and growth of perfect treatments for patients globally. Key
Challenges Off-target
Toxicity Related Hurdles to Act as a Challenge Antibody
drug conjugates witness several challenges which restrict their complete
potential. Drug resistance is a main issue, as the cancer cells can accept to
oppose the effects of cytotoxic payloads. Off-target toxicity also has a
problem, with the linkers that causes the unintended release of payloads in the
non-tumorous tissues which leads to bad effects. There
is restricted tumor entry that further limits drug delivery of ADC that
integrates an payload, antibody and an linker to add the challenges in both
clinical sues and preclinical studies. Technological
Advancements in the U.S. Antibody Drug Conjugates Market Immunostimulatory
antibody conjugates are a novel class of ADACS that mixes immune stimulators
with tumor -focusing antibodies in order to operate the immune system in the
tumor microenvironment, which develops anti-tumor effects. The
working of ISACs includes the particular delivery of immune agonists to the
tumor site who have activated antigen-presenting cells (APCs) and other immune
cells to develop immune feedback and make immune memory. For
instance, BDC-1001, which is an HER2 -that focuses on ISAC conjugated having
the TLR7/8 agonists that has displayed some perfect acceptability and some
anti-tumor activity in the clinical trials. Insight
into an FDA checked Antibody Drug Conjugates for the Cancer Therapy: •
There is an ADC which consists of an anti-HER2 antibody, which is a protease
cleavable tetrapeptide -dependent liker and the DXd as the drug payload . DXd
is a novel exatecan derivative which is crafted using the Daiichi Sankyo’s
proprietary art by using the ADC Technology. It
is linked with the camptothecin class of drug payload that causes their cryptic
effects by developing topoisomerase enzyme (TOP). (Source: https://pmc.ncbi.nlm.nih.gov) U.S.
Antibody Drug Conjugates Market Report Coverage Report Attribute Key Statistics Market Size in 2025 USD 7.54 Billion Market Size in 2026 USD 7.92 Billion Market Size in 2030 USD 9.62 Billion Market Size in 2032 USD 10.61 Billion Market Size by 2034 USD 11.70 Billion Growth rate from 2025 to
2034 CAGR of 5% Base Year 2024 Forecast Period 2025 to 2034 Segments Covered By Application, By Technology,
By Product, By Target Companies Covered ADC Therapeutics SA,
Takeda Pharmaceutical Company Ltd., Astellas Pharma, Inc., AstraZeneca Plc,
GlaxoSmithKline Plc, F. Hoffmann-La Roche Ltd., Daiichi Sankyo Company Ltd.,
Pfizer, Inc., Gilead Sciences, Inc., Sutro Biopharma
For
Orders or Inquiries, Don’t Hesitate to Reach Out: sales@visionresearchreports.com U.S.
Antibody Drug Conjugates Market Segmentation Analysis Application
Analysis Why
did the Breast Cancer Segment Dominated the U.S. Antibody Drug Conjugates
Market? Breast
cancer has dominated the U.S antibody drug conjugates market in 2024 as T-DM1 was the initial
ADC officially used for breast cancer depending on reported perfection over
lapatinib plus the capecitabine in the current study. T-DM1 has been globally
chosen as the sophisticated second-line diagnosis for HER2-positive breast
cancer since the year 2013. Hence,
data from the current DESTINY -Breast03 study which has shown reliable outcomes
with T-Dxd. Particularly, the medium overall survival (OS) was 55.6 months for
the T-DxD groups as compared to 42.7 months with the T-DM1 group. It is the
perfect performance in the DESTINY-Breast03 study as the T-DXd is currently the
selected choice for second-line treatment of HER2+ high level breast cancer. With
instance to this, •
Arvians and Pfizer current has revealed in detail outcomes form the Phase 3
VERITAC-2 Clinical trial named (NCT05654623) who is checking vepdegestrant monotherapy
as compared to fulvestrant in adults that estrogen receptor -positive and the
human epidermal growth factor receptor -2 negative. (Source: https://www.pfizer.com) The
urothelial and bladder cancer segment is predicted to rise at the fastest rate
in U.S. antibody drug conjugates market. Antibody drug conjugates have updated
the diagnosis scenario for high level bladder cancer that specifically
trastuzumab deruxtecan and the enfortumab vedotin that focuses on the Nectin-4
and human spiderman development receptor 2 respectively. These ADCs have
displayed a substantial smoothness that improves the existence in patients that
have developed after the chemotherapy and immunotherapy. Also,the imaging play
a crucial role in ADC-based therapy that streches beyond diagnosis and staging
to assessing treatment feedback that detects recurrence and checking the
toxicity too. Product
Analysis Why
did the Kadcyla Segment Dominated the U.S. Antibody Drug Conjugates Market? The
kadcyla segment has dominated the market in 2024 as it is an approved antibody-drug
conjugate which is crafted to serve with potent chemotherapy directly to
HER2-positive cancer cells that are capably limiting and toxic too with respect
to healthy problems. It has integrated two anti-cancer characteristics like
HER2 which focuses on the targeting properties of trastuzumab which is an
active ingredient in terms of Herceptin and the chemotherapy agent CM1. The
Enhertu segment is predicted to rise at the fastest rate in U.S. antibody drug
conjugates market. Enhertu
is an prescription drug that is utilised to treat adults who has HER2-low
breast cancer with cannot be avoided by surgery or that has spread to other
spaces of the body (metastatic) and who have received an advanced chemotherapy
too for te metastatic disease or the who has disease which has come back during
or within the 6 months of completing adjuvant chemotherapy after the surgery. With
instance to this, •
In April 2024, AstraZeneca and Daiichi Sankyo Enhertu has officially approved
in the U.S. for the diagnosis of adult patients with unrepeatable or metastatic
HER2 -positive solid tumors that has received prior systematic treatment and
have no satisfied alteration diagnosis options. (Source: https://www.astrazeneca.com) Target
Analysis Why
did the HER2 Segment Dominated the U.S. Antibody Drug Conjugates Market? HER2
segment has dominated the U.S. antibody drug conjugate industry as HER2
-positive breast cancer has been on top success story in focussed oncology that is specifically with
the growth of antibody -drug conjugates(ADCs). Such agents integrate the
particulars of monoclonal antibodies with the cytotoxic
capability of chemotherapeutic payloads. Trastuzumab
emtansine (T-DM1) and trastuzumab deruxtecan (t-dXd) are two such ADcs who have
updated diagnosis paradigms, specifically in advanced disease. Hence, these
struggles in order toxic ADCS into earlier treatment settings like as the
adjuvant phase and the neoadjuvant have aligned with mixed outcomes. The
CD22 segment is predicted to rise at the fastest rate in U.S. antibody drug conjugates
market. Cd22
is a crucial target for the antibody drug conjugates that are crafted to
diagnose B-cell malignancies like as particular lymphomas and the leukemia.It's
smooth as a target because of ots lineage -particular expression on the B-cells
and its fast internalisation upon the -antibody binding that enables ADCs to
serve with cytotoxic drug directly to the cancer cells. When
an anti-CD22 antibody combines to the CD22 receptor on the surface of a cancer
cell, then the anti-body receptor that is complicated quickly is taken inside
the cell. So,this procedure is important for serving the cryptotoxic drug that
payloads directly to its intended target. Technology
Analysis How
did the Cleavable linker Technology Segment Dominated the U.S. Antibody Drug Conjugates
Market? The
cleavable linker technology has dominated the market in 2024 as antibody-drug conjugates
(ADCs) are updating cancer therapy by integrating the targeting accuracy of the
monoclonal antibodies with the accuracy of cytotoxic drugs, this enables for
selective tumor cells by killing and lowering down the systemic toxicity which
serves and smooth treatment options for the patients with challenging-to-treat
cancers. The
linker controls when and where the cytotoxic drugs are being generated, which
makes it one of the most complicated design elements in an ADC. Linkers should
be stable during the circulation, but yellow smooth payload has been released
once inside the tumor cell. Cleavable linkers develop payload release
smoothness inside the cancer cells that has non-cleanable linkers which serve
superior systematic stability, The selection relies on the tumor type, wanted
therapeutic result and the payload characteristics. The
payload technology segment is predicted to rise at the fastest rate in the U.S.
antibody drug conjugates market. It is a developing advancement in terms of ADC technology
in the growth of dual-payload ADCs that includes two distinct cytotoxic agents
in the single conjugate. This method's goal is to solve tumor heterogeneity and
the drug opposition by continuously focusing on the several cellular pathways.
The Dual-payload ADCs can be crafted to serve drugs that have supplementary
working of action, capable of developing antitumor activity and solving the
resistance to single -magnet therapies. Need
a Tailored Version of the Report? | Get Customization Options Here: https://www.visionresearchreports.com/report/customization/41773 Recent
Developments in the U.S. Antibody Drug Conjugates Market •
In February 2025, Summit Therapeutics has disclosed a clinical partnership with
the Pfizer Inc to check a novel, ivonescimab and investigational PD-1 / VEGF
which is bispecific antibody in integration with many of Pfizer’s antibody drug
conjugates (ADC) across several solid tumor settings. (Source: https://www.businesswire.com) •
In September 2025, Glenmark Pharmaceuticals subsidiary has penetrated into the
USD1.1 billion partnership with Hengrui Pharma for the latest rights to
Trastuzumab Rezetecane, which is an future -generation HER2- that focuses on
antibody drug conjugates. (Source: https://scanx.trade) •
In June 2025, NextCure Inc, which is a clinical stage biopharmaceutical
organisation who is loyal to exploring and making novel, first-in-class and
best-in-class therapies in order to treat cancer and the Simcere Zaiming ,which
is an oncology -focused biopharmaceutical company and an subsister of the
Simcere Pharmaceutical Group Ltd. (Source: https://www.globenewswire.com) •
In August 2025, Systimmune INc, which is a clinical -stage biotechnology
company and the Bristol Myers Squibb currently had revealed that the U.S Food
and Drug Administration has approved the cutting-edge Therapy Designation for
the izalontamab brengitecan for the diagnosis of locally high level or
metastatic non -small cell lung cancer (NSCLC). (Source: https://www.prnewswire.com) Browse
More Insights: •
Drug Screening Market: https://www.visionresearchreports.com/drug-screening-market/41415 •
Antibody Drug Conjugates Market: https://www.visionresearchreports.com/antibody-drug-conjugates-market/41295 •
Antiemetics Drugs Market: https://www.visionresearchreports.com/antiemetics-drugs-market/41038 •
U.S. Drug Utilization Management Market: https://www.visionresearchreports.com/us-drug-utilization-management-market/41416 •
Ultomiris Drug Market: https://www.visionresearchreports.com/ultomiris-drug-market/41718 •
Generic Drugs Market: https://www.visionresearchreports.com/generic-drugs-market/41283 •
U.S. Ultomiris Drug Market: https://www.visionresearchreports.com/us-ultomiris-drug-market/41720 •
Psychedelic Drugs Market: https://www.visionresearchreports.com/psychedelic-drugs-market/41096 Top
Companies in the U.S. Antibody Drug Conjugates Market •
Takeda Pharmaceutical Company Ltd •
ADC Therapeutics SA •
GlaxoSmithKline Plc •
Daiichi Sankyo Company Ltd •
Pfizer, Inc •
F. Hoffman-La Roche Ltd •
Gilead Sciences, Inc •
Sutro Biopharma U.S. Antibody Drug Conjugates Market
Segmentation By
Application •
Blood Cancer • Leukemia • Lymphoma • Multiple Myeloma •
Breast Cancer •
Urothelial Cancer & Bladder Cancer •
Other Cancer By
Product •
Kadcyla •
Enhertu •
Adcetris •
Padcev •
Trodelvy •
Polivy •
Others By
Target •
HER2 •
CD22 •
CD30 •
Others By
Technology •
Type • Cleavable Linker • Non-cleavable Linker • Linkerless •
Linker Technology Type • VC • Sulfo-SPDB • VA • Hydrazone • Others •
Payload Technology • MMAE • MMAF • DM4g • Camptothecin • Others Instant
Delivery Available | Purchase This Exclusive Research Report Now: https://www.visionresearchreports.com/report/checkout/41773 You can place an order or ask any questions,
please feel free to contact at: sales@visionresearchreports.com About
Us Vision
Research Reports is a premier service provider offering strategic market
insights and solutions that go beyond traditional surveys. We specialize in
actionable market research, delivering in-depth qualitative insights and
strategies to global industry leaders and executives, helping them navigate
future uncertainties. Our offerings include consulting services, syndicated
market studies, and bespoke research reports. We
are committed to excellence in qualitative market research, fostering a team of
experts with deep industry knowledge. Our goal is to help clients understand
both current and future market trends, empowering them to expand their
portfolios and achieve their business objectives with the right guidance. Web: https://www.visionresearchreports.com Our
Trusted Data Partners Precedence Research | Statifacts | Nova One Advisor For
Latest Update Follow Us: LinkedIn
• Drug of Abuse Testing Services Market: https://www.visionresearchreports.com/drug-of-abuse-testing-services-market/41255
• Drug Discovery Market: https://www.visionresearchreports.com/drug-discovery-market/41160




