The Myelofibrosis Treatment Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.75 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 12.3% from 2025 to 2032. Myelofibrosis is a rare bone marrow disorder that disrupts the body’s normal process of blood cell production. It occurs when fibrous or scar tissue forms within the bone marrow, leading to scarring that impairs its ability to generate adequate blood cells. Classified as a type of chronic leukemia, myelofibrosis significantly affects healthy blood formation.
Request Sample Report: https://www.coherentmarketinsights.com/insight/request-sample/721
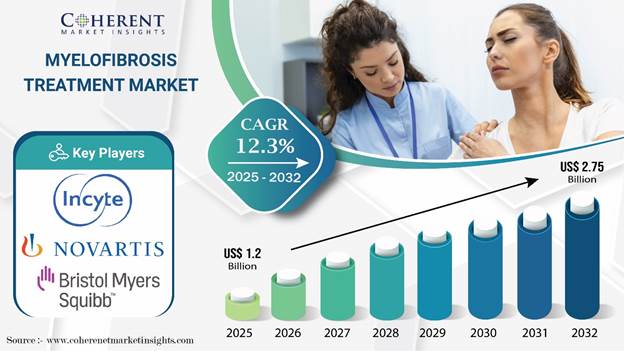
Global Myelofibrosis Treatment Market Key Takeaways
According to Coherent Market Insights (CMI), the global myelofibrosis treatment market size is projected to expand at a CAGR of 12.3% during the forecast period, reaching USD 2.75 Bn by 2032, up from USD 1.2 Bn in 2025.
JAK inhibitors continue to remain a highly sought-after treatment for myelofibrosis, with the target segment expected to account for 45% of the market share in 2025.
Based on route of administration, oral segment is set to account for a prominent myelofibrosis treatment market share in 2025.
North America is anticipated to account for two-fifths of the global myelofibrosis treatment industry share in 2025.
Asia Pacific, with an estimated CAGR of 14%, is expected to emerge as the most lucrative market for pharmaceutical companies during the forecast period.
Rising Prevalence of Myelofibrosis Spurring Market Growth
Coherent Market Insights’ new myelofibrosis treatment market analysis offers insights into major factors fueling industry growth. Increasing prevalence of myelofibrosis is one such prominent growth driver.
The global incidence of myelofibrosis is rising significantly, partly due to an aging global population and improved awareness and diagnosis. This is driving demand for effective myelofibrosis treatments, and the trend will likely continue in the coming years.
Immediate Delivery Available | Buy This Premium Research Report: https://www.coherentmarketinsights.com/insight/buy-now/721
High Treatment Costs and Side Effects Limiting Market Growth
The global myelofibrosis treatment market outlook appears promising, owing to rising myelofibrosis cases. However, high treatment costs and side effects might slow down market growth to some extent during the projection period.
Advanced myelofibrosis treatments, such as JAK inhibitors, are very costly. For example, the average yearly list price of Jakafi in the U.S. is about $17,150. This can limit patient access, especially in low- and middle-income regions, thereby dampening overall myelofibrosis treatment market demand.
In addition, long-term use of JAK inhibitors can cause side effects such as blood abnormalities, infections, and skin reactions. This may impact patient adherence and quality of life, sometimes resulting in treatment discontinuation.
Also Read: Leukemia Therapeutics Market Size, Share, Trends & Opportunities for 2025-2032
Advancements in Targeted Therapies Creating Growth Prospects
Targeted therapies, especially Janus kinase (JAK) inhibitors like ruxolitinib, fedratinib, pacritinib, and momelotinib, are revolutionizing treatment options for myelofibrosis patients. These therapies have demonstrated efficacy in reducing spleen size and alleviating symptoms.
They have the tendency to significantly improve patient outcomes. Growing adoption of these therapies is expected to create lucrative growth opportunities for myelofibrosis treatment manufacturers during the forthcoming period.
Emerging Myelofibrosis Treatment Market Trends
Combination therapies are gaining traction in myelofibrosis management. For instance, using ruxolitinib together with pelabresib has shown better results in clinical trials. These combinations are designed to help patients who do not respond well to JAK inhibitors or face side effects. The growing use of such combination therapies is likely to drive the growth of the myelofibrosis treatment market.
Ongoing product launches and approvals are contributing to market expansion. Leading players are striving to launch new products as well as receive approvals from regulatory bodies. For example, GSK's drug Omjjara recently received approval in Japan for the treatment of myelofibrosis.
Strategies like partnerships, acquisitions, and collaborations are becoming popular in the myleofibrosis treatment landscape. Many pharmaceutical companies are partnering with healthcare organizations to enhance myelofibrosis treatment accessibility. For instance, Incyte Corporation's recent collaboration with EuroBloodNet improved treatment access in Eastern Europe.
Also Read: Cancer Drugs Market Analysis & Forecast for 2025-2032
Analyst’s View
“The global myelofibrosis treatment industry is set to exhibit robust growth, owing to rising diagnosed cases of myelofibrosis, advancements in targeted therapies, growing popularity of combination therapies, and ongoing new product launches and approvals,” said a senior CMI analyst.
Current Events and Their Impact on the Myelofibrosis Treatment Market
|
Event |
Description and Impact |
|
Regulatory Advances in JAK Inhibitor Approvals |
o Impact: This allows novel myelofibrosis therapies to enter the market faster, reducing development timelines and providing patients with quicker access to new treatments. |
|
Technological Advances in Precision Medicine |
|
|
Strategic Pharmaceutical Industry Consolidation |
|
Competitor Insights
Key companies in myelofibrosis treatment market report include:
- Novartis AG
- Incyte Corporation
- Bristol-Myers Squibb Company
- Sanofi S.A.
- Pfizer Inc.
- CTI BioPharma Corp.
- Eli Lilly and Company
- Geron Corporation
- AbbVie Inc.
- Aclaris Therapeutics
Key Developments
· In March 2025, Mount Sinai’s Phase 3 trial showed that a new combination of medicines, pelabresib and ruxolitinib, could help improve outcomes for patients with myelofibrosis who hadn’t been treated before.
· In January 2024, the European Commission granted marketing authorization for Omjjara (momelotinib). This makes Omjjara the first EU-approved medicine for treating enlarged spleen or related symptoms in adults with myelofibrosis and moderate to severe anemia.
Request For Customization: https://www.coherentmarketinsights.com/insight/request-customization/721
Market Segmentation
- By Treatment Type: JAK Inhibitors, Immunomodulatory Agents, Chemotherapy, Stem Cell Transplantation, Others
- By Route of Administration: Oral, Injectable, Others
- By End-User: Hospitals, Specialty Clinics, Homecare Settings, Others
Also Read: Allogenic Stem Cell Transplantation Market Outlook for 2025-2032
Our Trusted Partners:
Worldwide Market Reports, Coherent MI, Stratagem Market Insights
Get Recent News:
https://www.coherentmarketinsights.com/news
About Us:
Coherent Market Insights leads into data and analytics, audience measurement, consumer behaviors, and market trend analysis. From shorter dispatch to in-depth insights, CMI has exceled in offering research, analytics, and consumer-focused shifts for nearly a decade. With cutting-edge syndicated tools and custom-made research services, we empower businesses to move in the direction of growth. We are multifunctional in our work scope and have 450+ seasoned consultants, analysts, and researchers across 26+ industries spread out in 32+ countries.
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd,
U.S.: + 12524771362
U.K.: +442039578553
AUS: +61-8-7924-7805
INDIA: +91-848-285-0837
✉ Email: sales@coherentmarketinsights.com

